Predicting individual-specific cardiotoxicity responses induced by tyrosine kinase inhibitors
- PMID: 37101545
- PMCID: PMC10123273
- DOI: 10.3389/fphar.2023.1158222
Predicting individual-specific cardiotoxicity responses induced by tyrosine kinase inhibitors
Abstract
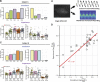
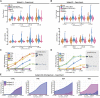
Introduction: Tyrosine kinase inhibitor drugs (TKIs) are highly effective cancer drugs, yet many TKIs are associated with various forms of cardiotoxicity. The mechanisms underlying these drug-induced adverse events remain poorly understood. We studied mechanisms of TKI-induced cardiotoxicity by integrating several complementary approaches, including comprehensive transcriptomics, mechanistic mathematical modeling, and physiological assays in cultured human cardiac myocytes. Methods: Induced pluripotent stem cells (iPSCs) from two healthy donors were differentiated into cardiac myocytes (iPSC-CMs), and cells were treated with a panel of 26 FDA-approved TKIs. Drug-induced changes in gene expression were quantified using mRNA-seq, changes in gene expression were integrated into a mechanistic mathematical model of electrophysiology and contraction, and simulation results were used to predict physiological outcomes. Results: Experimental recordings of action potentials, intracellular calcium, and contraction in iPSC-CMs demonstrated that modeling predictions were accurate, with 81% of modeling predictions across the two cell lines confirmed experimentally. Surprisingly, simulations of how TKI-treated iPSC-CMs would respond to an additional arrhythmogenic insult, namely, hypokalemia, predicted dramatic differences between cell lines in how drugs affected arrhythmia susceptibility, and these predictions were confirmed experimentally. Computational analysis revealed that differences between cell lines in the upregulation or downregulation of particular ion channels could explain how TKI-treated cells responded differently to hypokalemia. Discussion: Overall, the study identifies transcriptional mechanisms underlying cardiotoxicity caused by TKIs, and illustrates a novel approach for integrating transcriptomics with mechanistic mathematical models to generate experimentally testable, individual-specific predictions of adverse event risk.
Keywords: IPSC-CM cardiomyocytes; cardiotoxicity; kinase inhibitor; mRNA sequencing; mRNASeq; mathematical model.
Copyright © 2023 Shim, Xiong, Dhanan, Dariolli, Azeloglu, Hu, Jayaraman, Schaniel, Birtwistle, Iyengar, Dubois and Sobie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Charwat V., Charrez B., Siemons B. A., Finsberg H., Jaeger K. H., Edwards A. G., et al. (2022). Validating the arrhythmogenic potential of high-intermediate-and low-risk drugs in a human-induced pluripotent stem cell-derived cardiac microphysiological system. ACS Pharmacol. Transl. Sci. 5, 652–667. 10.1021/acsptsci.2c00088 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

