Ferritin: A Promising Nanoreactor and Nanocarrier for Bionanotechnology
- PMID: 37101573
- PMCID: PMC10114856
- DOI: 10.1021/acsbiomedchemau.2c00003
Ferritin: A Promising Nanoreactor and Nanocarrier for Bionanotechnology
Abstract
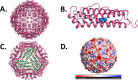
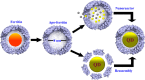
The essence of bionanotechnology lies in the application of nanotechnology/nanomaterials to solve the biological problems. Quantum dots and nanoparticles hold potential biomedical applications, but their inherent problems such as low solubility and associated toxicity due to their interactions at nonspecific target sites is a major concern. The self-assembled, thermostable, ferritin protein nanocages possessing natural iron scavenging ability have emerged as a potential solution to all the above-mentioned problems by acting as nanoreactor and nanocarrier. Ferritins, the cellular iron repositories, are hollow, spherical, symmetric multimeric protein nanocages, which sequester the excess of free Fe(II) and synthesize iron biominerals (Fe2O3·H2O) inside their ∼5-8 nm central cavity. The electrostatics and dynamics of the pore residues not only drives the natural substrate Fe2+ inside ferritin nanocages but also uptakes a set of other metals ions/counterions during in vitro synthesis of nanomaterial. The current review aims to report the recent developments/understanding on ferritin structure (self-assembly, surface/pores electrostatics, metal ion binding sites) and chemistry occurring inside these supramolecular protein cages (protein mediated metal ion uptake and mineralization/nanoparticle formation) along with its surface modification to exploit them for various nanobiotechnological applications. Furthermore, a better understanding of ferritin self-assembly would be highly useful for optimizing the incorporation of nanomaterials via the disassembly/reassembly approach. Several studies have reported the successful engineering of these ferritin protein nanocages in order to utilize them as potential nanoreactor for synthesizing/incorporating nanoparticles and as nanocarrier for delivering imaging agents/drugs at cell specific target sites. Therefore, the combination of nanoscience (nanomaterials) and bioscience (ferritin protein) projects several benefits for various applications ranging from electronics to medicine.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Rohela G. K.; Srinivasulu Y.; Rathore M. S. A Review Paper on Recent Trends in Bio-nanotechnology: Implications and Potentials. Nanoscience & Nanotechnology-Asia 2018, 9 (1), 12–20. 10.2174/2210681208666171204163015. - DOI
-
- Englebienne P.; Hoonacker A. V. Bionanotechnology: The Science of Revealing Life with Nanostructures. Current Nanoscience 2005, 1 (2), 97–106. 10.2174/1573413054065330. - DOI
Publication types
LinkOut - more resources
Full Text Sources
