Radical SAM Enzymes Involved in Tetrapyrrole Biosynthesis and Insertion
- PMID: 37101575
- PMCID: PMC10114771
- DOI: 10.1021/acsbiomedchemau.1c00061
Radical SAM Enzymes Involved in Tetrapyrrole Biosynthesis and Insertion
Abstract
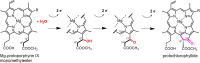
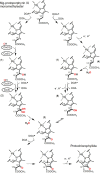
The anaerobic biosyntheses of heme, heme d 1, and bacteriochlorophyll all require the action of radical SAM enzymes. During heme biosynthesis in some bacteria, coproporphyrinogen III dehydrogenase (CgdH) catalyzes the decarboxylation of two propionate side chains of coproporphyrinogen III to the corresponding vinyl groups of protoporphyrinogen IX. Its solved crystal structure was the first published structure for a radical SAM enzyme. In bacteria, heme is inserted into enzymes by the cytoplasmic heme chaperone HemW, a radical SAM enzyme structurally highly related to CgdH. In an alternative heme biosynthesis route found in archaea and sulfate-reducing bacteria, the two radical SAM enzymes AhbC and AhbD catalyze the removal of two acetate groups (AhbC) or the decarboxylation of two propionate side chains (AhbD). NirJ, a close homologue of AhbC, is required for propionate side chain removal during the formation of heme d 1 in some denitrifying bacteria. Biosynthesis of the fifth ring (ring E) of all chlorophylls is based on an unusual six-electron oxidative cyclization step. The sophisticated conversion of Mg-protoporphyrin IX monomethylester to protochlorophyllide is facilitated by an oxygen-independent cyclase termed BchE, which is a cobalamin-dependent radical SAM enzyme. Most of the radical SAM enzymes involved in tetrapyrrole biosynthesis were recognized as such by Sofia et al. in 2001 (Nucleic Acids Res.2001, 29, 1097-1106) and were biochemically characterized thereafter. Although much has been achieved, the challenging tetrapyrrole substrates represent a limiting factor for enzyme/substrate cocrystallization and the ultimate elucidation of the corresponding enzyme mechanisms.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Mehta A. P.; Abdelwahed S. H.; Mahanta N.; Fedoseyenko D.; Philmus B.; Cooper L. E.; Liu Y.; Jhulki I.; Ealick S. E.; Begley T. P. Radical S-adenosylmethionine (SAM) enzymes in cofactor biosynthesis: a treasure trove of complex organic radical rearrangement reactions. J. Biol. Chem. 2015, 290 (7), 3980–3986. 10.1074/jbc.R114.623793. - DOI - PMC - PubMed
-
- Grimm B., Porra R. J., Rudiger W., Scheer H., Eds. Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Advances in Photosynthesis and Respiration; Springer: The Netherlands, 2006; Vol. 25.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
