Bacterial Concentrations and Water Turbulence Influence the Importance of Conjugation Versus Phage-Mediated Antibiotic Resistance Gene Transfer in Suspended Growth Systems
- PMID: 37101581
- PMCID: PMC10114721
- DOI: 10.1021/acsenvironau.1c00027
Bacterial Concentrations and Water Turbulence Influence the Importance of Conjugation Versus Phage-Mediated Antibiotic Resistance Gene Transfer in Suspended Growth Systems
Abstract
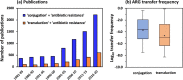
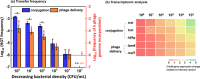
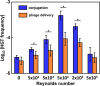
Despite the abundance of phage-borne antibiotic resistance genes (ARGs) in the environment, the frequency of ARG propagation via phage-mediated transduction (relative to via conjugation) is poorly understood. We investigated the influence of bacterial concentration and water turbulence level [quantified as Reynold's number (Re)] in suspended growth systems on the frequency of ARG transfer by two mechanisms: delivery by a lysogenic phage (phage λ carrying gentamycin-resistance gene, genR) and conjugation mediated by the self-transmissible plasmid RP4. Using Escherichia coli (E. coli) as the recipient, phage delivery had a comparable frequency (1.2 ± 0.9 × 10-6) to that of conjugation (1.1 ± 0.9 × 10-6) in suspensions with low cell concentration (104 CFU/mL) and moderate turbulence (Re = 5 × 104). Turbulence affected cell (or phage)-to-cell contact rates and detachment (due to shear force), and thus, it affected the relative importance of conjugation versus phage delivery. At 107 CFU/mL, no significant difference was observed between the frequencies of ARG transfer by the two mechanisms under quiescent water conditions (2.8 ± 0.3 × 10-5 for conjugation vs 2.2 ± 0.5 × 10-5 for phage delivery, p = 0.19) or when Re reached 5 × 105 (3.4 ± 1.5 × 10-5 for conjugation vs 2.9 ± 1.0 × 10-5 for phage delivery, p = 0.52). Transcriptomic analysis of genes related to conjugation and phage delivery and simulation of cell (or phage)-to-cell collisions at different Re values corroborate that the importance of phage delivery relative to conjugation increases under either quiescent or turbulent conditions. This finding challenges the prevailing view that conjugation is the dominant ARG transfer mechanism and underscores the need to consider and mitigate potential ARG dissemination via transduction.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- O’Neill J.Review on Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; HM Government: London, 2014.
-
- Nelson R. E.; Hatfield K. M.; Wolford H.; Samore M. H.; Scott R. D.; Reddy S. C.; Olubajo B.; Paul P.; Jernigan J. A.; Baggs J. National estimates of healthcare costs associated with multidrug-resistant bacterial infections among hospitalized patients in the United States. Clin. Infect. Dis. 2021, 72, S17–S26. 10.1093/cid/ciaa1581. - DOI - PMC - PubMed
-
- Vikesland P. J.; Pruden A.; Alvarez P. J. J.; Aga D.; Bürgmann H.; Li X.-d.; Manaia C. M.; Nambi I.; Wigginton K.; Zhang T.; Zhu Y.-G. Toward a comprehensive strategy to mitigate dissemination of environmental sources of antibiotic resistance. Environ. Sci. Technol. 2017, 51, 13061–13069. 10.1021/acs.est.7b03623. - DOI - PubMed
-
- von Wintersdorff C. J.; Penders J.; van Niekerk J. M.; Mills N. D.; Majumder S.; van Alphen L. B.; Savelkoul P. H.; Wolffs P. F. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 7, 173.10.3389/fmicb.2016.00173. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
