Palbociclib in Acute Leukemias With KMT2A-rearrangement: Results of AMLSG 23-14 Trial
- PMID: 37101762
- PMCID: PMC10125418
- DOI: 10.1097/HS9.0000000000000877
Palbociclib in Acute Leukemias With KMT2A-rearrangement: Results of AMLSG 23-14 Trial
Conflict of interest statement
VIG: Consulting or advisory board membership: Jazz Pharmaceuticals; Speakers bureau: Abbvie, Janssen, Pfizer; Travel or accommodation expenses: AbbVie. PP: Consulting or advisory board membership: AbbVie, Agios, Astellas, Astex, Bristol-Meyers Squibb, Celgene, Jazz, Novartis, Otsuka, Pfizer, Sunesis; Speakers bureau: AbbVie, Agios, Astellas, Bristol-Meyers Squibb, Celgene, Jazz, Novartis, Pfizer; Travel or accommodation expenses: AbbVie, Amgen, Bristol-Meyers Squibb, Celgene, Janssen, Novartis, Takeda. RFS: Consulting or advisory board membership: Abbvie, Agios, Astellas, Celgene, Daiichi Sankyo, Hexal, Neovio Biotech, Novartis, Pfizer, Roche; research funding: Astellas, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Roche; speakers bureau, Astellas, Pfizer. SF: Consulting or advisory board membership: Bayer, Illumina, Roche; honoraria: Amgen, Eli Lilly, PharmaMar, Roche; research funding: AstraZeneca, Pfizer, PharmaMar, Roche; travel or accommodation expenses: Amgen, Eli Lilly, Illumina, PharmaMar, Roche. AK: Consulting, honoraria, advisory board membership: Roche; research funding: BMS, Roche. RW: Consulting or advisory board: Janssen, Novartis, BMS/Celgene, Amgen, Gilead, Pfizer, Sanofi, Takeda; research funding: Janssen, Sanofi; Honoraria: Abbvie, Sanofi, Takeda, Janssen, Amgen, BMS/Celgene, Gilead, Pfizer. JW: Consulting or advisory board membership: Novartis, Bristol Myers Squibb, Celgene, Amgen, Abbvie, Agios, Jazz, Pfizer, Sanofi, Sobi, Astellas. MdW: Research funding: Pfizer, Abbvie, Novartis, Astellas, Bristol Myers Squibb, AstraZeneca, MorphoSys; speakers honoraria: AstraZeneca, Janssen; travel or accommodation expenses: Astellas, Janssen. WF: Consulting or advisory board membership: AbbVie, Amgen, Celgene, Jazz Pharmaceuticals, MorphoSys AG, Novartis, Pfizer; Stemline and Clinigen; research funding: from Amgen and Pfizer; patents and royalties: Amgen; travel or accommodation expenses: Amgen, Daiichi Sankyo, Gilead, Jazz Pharmaceuticals, and Servier. MH: Consulting or advisory board: Abbvie, BMS/Celgene, Daiichi Sankyo, Jazz Pharmaceuticals, Novartis, Pfizer, Roche, Tolremo. Honoraria: Jazz Pharmaceuticals, Janssen, Novartis; research funding: Astellas, Bayer Pharma AG, BergenBio, Daiichi Sankyo, Jazz Pharmaceuticals, Karyopharm, Novartis, Pfizer, Roche. FT: Consulting or advisory board: Abbvie, Astellas, Bristol Myers Squibb/Celgene, Jazz Pharmaceuticals, Novartis, Pfizer. KD: Consulting or advisory board: Novartis, Celgene/BMS, Abbvie, Jazz Pharmaceuticals; research funding: Novartis; Celgene/BMS, Astellas, Agios, Kronos; Honoraria: Novartis, Celgene/BMS, Jazz Pharmaceuticals. HD: Consulting or advisory board: AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, BMS, Celgene, Daiichi Sankyo, Gilead, Janssen, Jazz, Novartis, Servier, Syndax; Clinical Research Funding to Institution: AbbVie, Agios, Amgen, Astellas, Bristol Myers Squibb, Celgene, Jazz Pharmaceuticals, Kronos Bio, Novartis, Pfizer. All the other authors have no conflicts of interest to disclose.
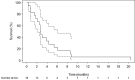
Figures
References
-
- Moorman AV. The clinical relevance of chromosomal and genomic abnormalities in B cell pre-cursor acute lymphoblastic leukaemia. Blood Rev. 2012;26:123–135. - PubMed
-
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 ELN recommendations from an international expert panel. Blood. 2022:blood.2022016867. Epub ahead of print. - PubMed
LinkOut - more resources
Full Text Sources