Controlling Amyloid Beta Peptide Aggregation and Toxicity by Protease-Stable Ligands
- PMID: 37101809
- PMCID: PMC10125337
- DOI: 10.1021/acsbiomedchemau.2c00067
Controlling Amyloid Beta Peptide Aggregation and Toxicity by Protease-Stable Ligands
Abstract
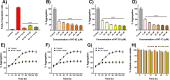
Polymerization of soluble amyloid beta (Aβ) peptide into protease-stable insoluble fibrillary aggregates is a critical step in the pathogenesis of Alzheimer's disease (AD). The N-terminal (NT) hydrophobic central domain fragment 16KLVFF20 plays an important role in the formation and stabilization of β-sheets by self-recognition of the parent Aβ peptide, followed by aggregation of Aβ in the AD brain. Here, we analyze the effect of the NT region inducing β-sheet formation in the Aβ peptide by a single amino acid mutation in the native Aβ peptide fragment. We designed 14 hydrophobic peptides (NT-01 to NT-14) by a single mutation at 18Val by using hydrophobic residues leucine and proline in the natural Aβ peptide fragment (KLVFFAE) and analyzed its effect on the formation of Aβ aggregates. Among all these peptides, NT-02, NT-03, and NT-13 significantly affected the Aβ aggregate formation. When the NT peptides were coincubated with the Aβ peptide, a significant reduction in β-sheet formation and increment in random coil content of Aβ was seen, confirmed by circular dichroism spectroscopy and Fourier transform infrared spectroscopy, followed by the reduction of fibril formation measured by the thioflavin-T (ThT) binding assay. The aggregation inhibition was monitored by Congo red and ThT staining and electron microscopic examination. Moreover, the NT peptides protect the PC-12 differentiated neurons from Aβ-induced toxicity and apoptosis in vitro. Thus, manipulation of the Aβ secondary structure with protease-stable ligands that promote the random coil conformation may provide a tool to control the Aβ aggregates observed in AD patients.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
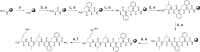








References
-
- Roher A. E.; Lowenson J. D.; Clarke S.; Woods A. S.; Cotter R. J.; Gowing E.; Ball M. J. Beta-Amyloid-(1-42) Is a Major Component of Cerebrovascular Amyloid Deposits: Implications for the Pathology of Alzheimer Disease. Proc. Natl. Acad. Sci. 1993, 90, 10836–10840. 10.1073/pnas.90.22.10836. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

