Influence of Cell Type on the Efficacy of Plasmonic Photothermal Therapy
- PMID: 37101851
- PMCID: PMC10125312
- DOI: 10.1021/acsnanoscienceau.2c00023
Influence of Cell Type on the Efficacy of Plasmonic Photothermal Therapy
Abstract
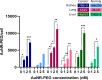
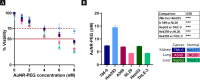
In plasmonic photothermal therapy (PPTT), illuminated gold nanoparticles are locally heated to produce selective damage in cells. While PPTT is expected to strongly depend on the cell line, available data are sparse and critical parameters remain unclear. To elucidate this pivotal aspect, we present a systematic study of diseased and nondiseased cells from different tissues to evaluate cytotoxicity, uptake of gold nanorods (AuNRs), and viability after PPTT. We identified differences in uptake and toxicity between cell types, linking AuNR concentrations to toxicity. Furthermore, the cell death mechanism is shown to depend on the intensity of the irradiated light and hence the temperature increase. Importantly, the data also underline the need to monitor cell death at different time points. Our work contributes to the definition of systematic protocols with appropriate controls to fully comprehend the effects of PPTT and build meaningful and reproducible data sets, key to translate PPTT to clinical settings.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Standardization of In Vitro Studies for Plasmonic Photothermal therapy.ACS Nanosci Au. 2023 Jul 17;3(5):347-352. doi: 10.1021/acsnanoscienceau.3c00011. eCollection 2023 Oct 18. ACS Nanosci Au. 2023. PMID: 37868227 Free PMC article. Review.
-
Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice.Proc Natl Acad Sci U S A. 2017 Apr 11;114(15):E3110-E3118. doi: 10.1073/pnas.1619302114. Epub 2017 Mar 29. Proc Natl Acad Sci U S A. 2017. PMID: 28356516 Free PMC article.
-
Targeting heat shock protein 70 using gold nanorods enhances cancer cell apoptosis in low dose plasmonic photothermal therapy.Biomaterials. 2016 Sep;102:1-8. doi: 10.1016/j.biomaterials.2016.06.017. Epub 2016 Jun 7. Biomaterials. 2016. PMID: 27318931
-
Preventing Metastasis Using Gold Nanorod-Assisted Plasmonic Photothermal Therapy in Xenograft Mice.Bioconjug Chem. 2022 Dec 21;33(12):2320-2331. doi: 10.1021/acs.bioconjchem.2c00011. Epub 2022 Feb 14. Bioconjug Chem. 2022. PMID: 35156818
-
Plasmonic photothermal therapy (PPTT) using gold nanoparticles.Lasers Med Sci. 2008 Jul;23(3):217-28. doi: 10.1007/s10103-007-0470-x. Epub 2007 Aug 3. Lasers Med Sci. 2008. PMID: 17674122 Review.
Cited by
-
Standardization of In Vitro Studies for Plasmonic Photothermal therapy.ACS Nanosci Au. 2023 Jul 17;3(5):347-352. doi: 10.1021/acsnanoscienceau.3c00011. eCollection 2023 Oct 18. ACS Nanosci Au. 2023. PMID: 37868227 Free PMC article. Review.
-
Blue-LED assisted Photodegradation kinetics of rhodamine-6G dye, enhanced anticancer activity and cleavage of plasmids using Au-ZnO nanocomposite.Heliyon. 2024 Dec 11;11(1):e41061. doi: 10.1016/j.heliyon.2024.e41061. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39801960 Free PMC article.
-
Effectiveness of Gold Nanorods of Different Sizes in Photothermal Therapy to Eliminate Melanoma and Glioblastoma Cells.Int J Mol Sci. 2023 Aug 27;24(17):13306. doi: 10.3390/ijms241713306. Int J Mol Sci. 2023. PMID: 37686114 Free PMC article.
References
-
- Bettaieb A.; Wrzal P. K.; Averill-bates D. A. Hyperthermia: Cancer Treatment and Beyond. Cancer Treat. Innov. Approaches 2013, 257–283. 10.5772/55795. - DOI
-
- Vilches C.; Quidant R.. Targeted hyperthermia with plasmonic nanoparticles. In Frontiers of Nanoscience, 1st ed.; Elsevier Ltd, 2020; Vol. 16, pp 307–352. 10.1016/B978-0-08-102828-5.00012-7. - DOI
LinkOut - more resources
Full Text Sources
