A new class of anti-proliferative activity and apoptotic inducer with molecular docking studies for a novel of 1,3-dithiolo[4,5- b]quinoxaline derivatives hybrid with a sulfonamide moiety
- PMID: 37101951
- PMCID: PMC10123497
- DOI: 10.1039/d3ra01635h
A new class of anti-proliferative activity and apoptotic inducer with molecular docking studies for a novel of 1,3-dithiolo[4,5- b]quinoxaline derivatives hybrid with a sulfonamide moiety
Abstract
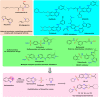
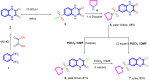
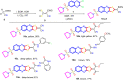
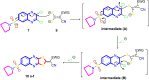
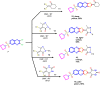
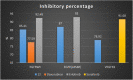
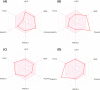
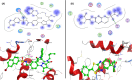
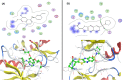
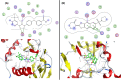
A new series of 6-(pyrrolidin-1-ylsulfonyl)-[1,3]dithiolo[4,5-b]quinoxaline-2-ylidines 10a-f, 12, 14, 16, and 18 were designed, synthesized, and evaluated for their in vitro anticancer activity. The structures of the novel compounds were systematically characterized by 1H NMR, 13C NMR, and elemental analysis. The synthesized derivatives were evaluated for their in vitro antiproliferative activity against three human cancer cell lines (HepG-2, HCT-116, and MCF-7) with more sensitivity to MCF-7. Moreover, three derivatives 10c, 10f, and 12 were the most promising candidates with sub-micromole values. These derivatives were further evaluated against MDA-MB-231, and the results displayed significant IC50 values ranging from 2.26 ± 0.1 to 10.46 ± 0.8 μM and showed low cellular cytotoxicity against WI-38. Surprisingly, the most active derivative 12 revealed sensitivity towards the breast cell lines MCF-7 (IC50 = 3.82 ± 0.2 μM) and MDA-MB-231 (IC50 = 2.26 ± 0.1 μM) compared with doxorubicin (IC50 = 4.17 ± 0.2 and 3.18 ± 0.1 M). Cell cycle analysis showed that compound 12 arrests and inhibits the growth of MCF-7 cells in the S phase with values of 48.16% compared with the untreated control 29.79% and exhibited a significantly higher apoptotic effect in MCF-7 with a value of 42.08% compared to control cell at 1.84%. Furthermore, compound 12 decreased Bcl-2 protein 0.368-fold and activation on pro-apoptotic genes Bax and P53 by 3.97 and 4.97 folds, respectively, in MCF-7 cells. Compound 12 exhibited higher inhibitory activity to EGFRWt, EGFRL858R, and VEGFR-2 with IC50 values (0.19 ± 0.009, 0.026 ± 0.001, and 0.42 ± 0.021 μM) compared with erlotinib (IC50 = 0.037 ± 0.002 and 0.026 ± 0.001 μM) and sorafenib (IC50 = 0.035 ± 0.002 μM). Finally, in silico ADMET prediction presented that 1,3-dithiolo[4,5-b]quinoxaline derivative 12 obeys the Lipinski rule of five and the Veber rule with no PAINs alarms and moderately soluble properties. Additionally, toxicity prediction revealed that compound 12 demonstrated inactivity to hepatotoxic carcinogenicity, immunotoxicity, mutagenicity, and cytotoxicity. Moreover, molecular docking studies showed good binding affinity with lower binding energy inside the active site of Bcl-2 (PDB: 4AQ3), EGFR (PDB: 1M17), and VEGFR (PDB: 4ASD).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Rahman M. M. Islam M. R. Akash S. Harun-Or-Rashid M. Ray T. K. Rahaman M. S. Islam M. Anika F. Hosain M. K. Aovi F. I. Hemeg H. A. Rauf A. Wilairatana P. Biomed. Pharmacother. 2022;153:113305. - PubMed
-
- Rahman M. M. Islam M. R. Akash S. Shohag S. Ahmed L. Supti F. A. Rauf A. Aljohani A. S. M. Al Abdulmonem W. Khalil A. A. Sharma R. Thiruvengadam M. Chem.-Biol. Interact. 2022;368:110198. - PubMed
-
- Siegel R. L. Miller K. D. Fuchs H. E. Jemal A. Cancer C. A. J. Clin. 2022;72:7–33. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

