Inactivation of p53 provides a competitive advantage to del(5q) myelodysplastic syndrome hematopoietic stem cells during inflammation
- PMID: 37102608
- PMCID: PMC10542836
- DOI: 10.3324/haematol.2022.282349
Inactivation of p53 provides a competitive advantage to del(5q) myelodysplastic syndrome hematopoietic stem cells during inflammation
Abstract
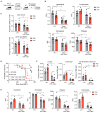
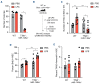
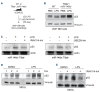
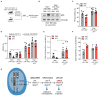
Inflammation is associated with the pathogenesis of myelodysplastic syndromes (MDS) and emerging evidence suggests that MDS hematopoietic stem and progenitor cells (HSPC) exhibit an altered response to inflammation. Deletion of chromosome 5 (del(5q)) is the most common chromosomal abnormality in MDS. Although this MDS subtype contains several haploinsufficient genes that impact innate immune signaling, the effects of inflammation on del(5q) MDS HSPC remains undefined. Utilizing a model of del(5q)-like MDS, inhibiting the IRAK1/4-TRAF6 axis improved cytopenias, suggesting that activation of innate immune pathways contributes to certain clinical features underlying the pathogenesis of low-risk MDS. However, low-grade inflammation in the del(5q)-like MDS model did not contribute to more severe disease but instead impaired the del(5q)-like HSPC as indicated by their diminished numbers, premature attrition and increased p53 expression. Del(5q)-like HSPC exposed to inflammation became less quiescent, but without affecting cell viability. Unexpectedly, the reduced cellular quiescence of del(5q) HSPC exposed to inflammation was restored by p53 deletion. These findings uncovered that inflammation confers a competitive advantage of functionally defective del(5q) HSPC upon loss of p53. Since TP53 mutations are enriched in del(5q) AML following an MDS diagnosis, increased p53 activation in del(5q) MDS HSPC due to inflammation may create a selective pressure for genetic inactivation of p53 or expansion of a pre-existing TP53-mutant clone.
Figures



References
-
- Stubbins RJ, Platzbecker U, Karsan A. Inflammation and myeloid malignancy: quenching the flame. Blood. 2022;140(10):1067-1074. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

