Caspofungin-Loaded Formulations for Treating Ocular Infections Caused by Candida spp
- PMID: 37102960
- PMCID: PMC10138186
- DOI: 10.3390/gels9040348
Caspofungin-Loaded Formulations for Treating Ocular Infections Caused by Candida spp
Abstract
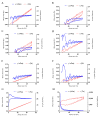
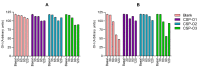
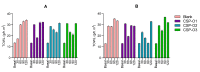
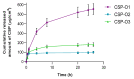
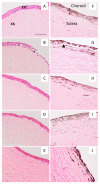
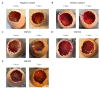
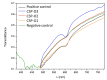
Fungal keratitis causes corneal blindness worldwide. The treatment includes antibiotics, with Natamycin being the most commonly used; however, fungal keratitis is difficult to treat, so alternative therapies are needed. In situ gelling formulations are a promising alternative; this type of formulation has the advantages of eye drops combined with the advantages of ointments. This study was designed to develop and characterize three formulations containing 0.5% CSP: CSP-O1, CSP-O2, and CSP-O3. CSP is an antifungal drug that acts against a diverse variety of fungi, and Poloxamer 407 (P407) is a polymer of synthetic origin that is able to produce biocompatible, biodegradable, highly permeable gels and is known to be thermoreversible. Short-term stability showed that formulations are best stored at 4 °C, and rheological analysis showed that the only formulation able to gel in situ was CSP-O3. In vitro release studies indicated that CSP-O1 releases CSP most rapidly, while in vitro permeation studies showed that CSP-O3 permeated the most. The ocular tolerance study showed that none of the formulations caused eye irritation. However, CSP-O1 decreased the cornea's transparency. Histological results indicate that the formulations are suitable for use, with the exception of CSP-O3, which induced slight structural changes in the scleral structure. All formulations were shown to have antifungal activity. In view of the results obtained, these formulations could be promising candidates for use in the treatment of fungal keratitis.
Keywords: antifungal activity; caspofungin; ocular drug delivery; ocular gels; ocular infection; poloxamer 407.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
New Formulations Loading Caspofungin for Topical Therapy of Vulvovaginal Candidiasis.Gels. 2021 Dec 12;7(4):259. doi: 10.3390/gels7040259. Gels. 2021. PMID: 34940319 Free PMC article.
-
Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies.Eur J Pharm Biopharm. 2017 May;114:119-134. doi: 10.1016/j.ejpb.2017.01.008. Epub 2017 Jan 24. Eur J Pharm Biopharm. 2017. PMID: 28126392
-
A stepwise optimization strategy to formulate in situ gelling formulations comprising fluconazole-hydroxypropyl-beta-cyclodextrin complex loaded niosomal vesicles and Eudragit nanoparticles for enhanced antifungal activity and prolonged ocular delivery.Asian J Pharm Sci. 2020 Sep;15(5):617-636. doi: 10.1016/j.ajps.2019.09.003. Epub 2019 Dec 24. Asian J Pharm Sci. 2020. PMID: 33193864 Free PMC article.
-
Clinical utility of caspofungin eye drops in fungal keratitis.Int J Antimicrob Agents. 2014 Aug;44(2):96-104. doi: 10.1016/j.ijantimicag.2014.04.008. Epub 2014 May 17. Int J Antimicrob Agents. 2014. PMID: 24933448 Review.
-
Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections.Drugs. 2009;69(3):361-92. doi: 10.2165/00003495-200969030-00010. Drugs. 2009. PMID: 19275278 Review.
Cited by
-
Evaluation of Olive Oil-Based Formulations Loaded with Baricitinib for Topical Treatment of Alopecia Areata.Pharmaceutics. 2025 Apr 5;17(4):475. doi: 10.3390/pharmaceutics17040475. Pharmaceutics. 2025. PMID: 40284470 Free PMC article.
-
Novel Gels for Post-Piercing Care: Evaluating the Efficacy of Pranoprofen Formulations in Reducing Inflammation.Gels. 2025 Apr 30;11(5):334. doi: 10.3390/gels11050334. Gels. 2025. PMID: 40422354 Free PMC article.
-
PLGA Nanoparticles Containing Natural Flavanones for Ocular Inflammation.Pharmaceutics. 2023 Dec 11;15(12):2752. doi: 10.3390/pharmaceutics15122752. Pharmaceutics. 2023. PMID: 38140093 Free PMC article.
-
A novel liposomal formulation for ocular delivery of caspofungin: an experimental study by quality by design-based approach.Ther Deliv. 2024;15(9):667-683. doi: 10.1080/20415990.2024.2379756. Epub 2024 Aug 5. Ther Deliv. 2024. PMID: 39101438 Free PMC article.
-
Layer-by-Layer Biopolymer-Coated Deformable Liposomes-In Situ Gel: A Hybrid Strategy for Enhanced Ocular Delivery of Itraconazole: In Vitro and In Vivo Appraisal.Gels. 2024 Dec 31;11(1):19. doi: 10.3390/gels11010019. Gels. 2024. PMID: 39851990 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

