Hyphal Growth in Trichosporon asahii Is Accelerated by the Addition of Magnesium
- PMID: 37102973
- PMCID: PMC10269644
- DOI: 10.1128/spectrum.04242-22
Hyphal Growth in Trichosporon asahii Is Accelerated by the Addition of Magnesium
Abstract
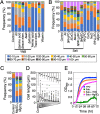
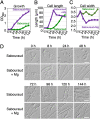
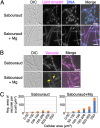
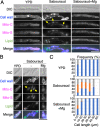
Fungal dimorphism involves two morphologies: a unicellular yeast cell and a multicellular hyphal form. Invasion of hyphae into human cells causes severe opportunistic infections. The transition between yeast and hyphal forms is associated with the virulence of fungi; however, the mechanism is poorly understood. Therefore, we aimed to identify factors that induce hyphal growth of Trichosporon asahii, a dimorphic basidiomycete that causes trichosporonosis. T. asahii showed poor growth and formed small cells containing large lipid droplets and fragmented mitochondria when cultivated for 16 h in a nutrient-deficient liquid medium. However, these phenotypes were suppressed via the addition of yeast nitrogen base. When T. asahii cells were cultivated in the presence of different compounds present in the yeast nitrogen base, we found that magnesium sulfate was a key factor for inducing cell elongation, and its addition dramatically restored hyphal growth in T. asahii. In T. asahii hyphae, vacuoles were enlarged, the size of lipid droplets was decreased, and mitochondria were distributed throughout the cell cytoplasm and adjacent to the cell walls. Additionally, hyphal growth was disrupted due to treatment with an actin inhibitor. The actin inhibitor latrunculin A disrupted the mitochondrial distribution even in hyphal cells. Furthermore, magnesium sulfate treatment accelerated hyphal growth in T. asahii for 72 h when the cells were cultivated in a nutrient-deficient liquid medium. Collectively, our results suggest that an increase in magnesium levels triggers the transition from the yeast to hyphal form in T. asahii. These findings will support studies on the pathogenesis of fungi and aid in developing treatments. IMPORTANCE Understanding the mechanism underlying fungal dimorphism is crucial to discern its invasion into human cells. Invasion is caused by the hyphal form rather than the yeast form; therefore, it is important to understand the mechanism of transition from the yeast to hyphal form. To study the transition mechanism, we utilized Trichosporon asahii, a dimorphic basidiomycete that causes severe trichosporonosis since there are fewer studies on T. asahii than on ascomycetes. This study suggests that an increase in Mg2+, the most abundant mineral in living cells, triggers growth of filamentous hyphae and increases the distribution of mitochondria throughout the cell cytoplasm and adjacent to the cell walls in T. asahii. Understanding the mechanism of hyphal growth triggered by Mg2+ increase will provide a model system to explore fungal pathogenicity in the future.
Keywords: Trichosporon asahii; fungal dimorphism; hyphal growth; magnesium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

