Synthesis and Antibiotic Activity of Chitosan-Based Comb-like Co-Polypeptides
- PMID: 37103382
- PMCID: PMC10143536
- DOI: 10.3390/md21040243
Synthesis and Antibiotic Activity of Chitosan-Based Comb-like Co-Polypeptides
Abstract
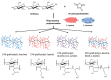
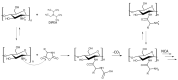
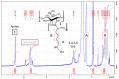
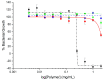
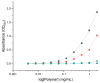
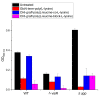
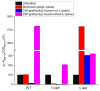
Infections caused by multidrug-resistant Gram-negative bacteria have been named one of the most urgent global health threats due to antimicrobial resistance. Considerable efforts have been made to develop new antibiotic drugs and investigate the mechanism of resistance. Recently, Anti-Microbial Peptides (AMPs) have served as a paradigm in the design of novel drugs that are active against multidrug-resistant organisms. AMPs are rapid-acting, potent, possess an unusually broad spectrum of activity, and have shown efficacy as topical agents. Unlike traditional therapeutics that interfere with essential bacterial enzymes, AMPs interact with microbial membranes through electrostatic interactions and physically damage cell integrity. However, naturally occurring AMPs have limited selectivity and modest efficacy. Therefore, recent efforts have focused on the development of synthetic AMP analogs with optimal pharmacodynamics and an ideal selectivity profile. Hence, this work explores the development of novel antimicrobial agents which mimic the structure of graft copolymers and mirror the mode of action of AMPs. A family of polymers comprised of chitosan backbone and AMP side chains were synthesized via the ring-opening polymerization of the N-carboxyanhydride of l-lysine and l-leucine. The polymerization was initiated from the functional groups of chitosan. The derivatives with random- and block-copolymer side chains were explored as drug targets. These graft copolymer systems exhibited activity against clinically significant pathogens and disrupted biofilm formation. Our studies highlight the potential of chitosan-graft-polypeptide structures in biomedical applications.
Keywords: N-carboxyanhydrides; antimicrobial peptides; chitosan; comb-like co-polypeptide; ring-opening polymerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

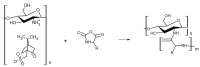



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

