Slipper Limpet (Crepidula fornicata) Shells Support In Vitro Osteogenesis of Human Adipose-Derived Stem Cells
- PMID: 37103387
- PMCID: PMC10142914
- DOI: 10.3390/md21040248
Slipper Limpet (Crepidula fornicata) Shells Support In Vitro Osteogenesis of Human Adipose-Derived Stem Cells
Abstract
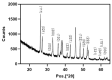
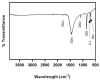
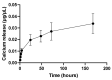
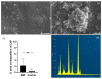
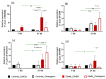
This study aimed to investigate a cost-effective alternative to man-made calcium phosphate ceramics for treating bone defects. The slipper limpet is an invasive species in European coastal waters, and its shells composed of calcium carbonate could potentially be a cost-effective source of bone graft substitutes. This research analyzed the mantle of the slipper limpet (Crepidula fornicata) shells to enhance in vitro bone formation. Discs machined from the mantle of C. fornicata were analyzed using scanning electron microscopy with energy dispersive spectroscopy (SEM-EDS), X-ray crystallography (XRD), Fourier-transform infrared spectroscopy (FT-IR) and profilometry. Calcium release and bioactivity were also studied. Cell attachment, proliferation, and osteoblastic differentiation (RT-qPCR and alkaline phosphatase activity) were measured in human adipose-derived stem cells grown on the mantle surface. The mantle material was mainly composed of aragonite and showed a sustained Ca2+ release at physiological pH. In addition, apatite formation was observed in simulated body fluid after three weeks, and the materials supported osteoblastic differentiation. Overall, our findings suggest the mantle of C. fornicata shows potential as a material for fabricating bone graft substitutes and structural biomaterials for bone regeneration.
Keywords: Crepidula fornicata; calcium carbonate; mantle; mesenchymal stem cells; osteogenesis; slipper limpet shells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
The invasive, non-native slipper limpet Crepidula fornicata is poorly adapted to sediment burial.Mar Pollut Bull. 2018 May;130:95-104. doi: 10.1016/j.marpolbul.2018.03.006. Epub 2018 Mar 20. Mar Pollut Bull. 2018. PMID: 29866575
-
Osteogenic differentiation of human bone marrow-derived mesenchymal stem cells is enhanced by an aragonite scaffold.Differentiation. 2019 May-Jun;107:24-34. doi: 10.1016/j.diff.2019.05.002. Epub 2019 May 22. Differentiation. 2019. PMID: 31152959
-
Zinc-coated carbonate apatite derived from avian eggshell for potential use as bone substitute. Part I: preparation and properties.Implant Dent. 2012 Jun;21(3):230-5. doi: 10.1097/ID.0b013e3182563ce5. Implant Dent. 2012. PMID: 22584418
-
In vitro evaluation of bioactive strontium-based ceramic with rabbit adipose-derived stem cells for bone tissue regeneration.J Mater Sci Mater Med. 2013 Dec;24(12):2831-44. doi: 10.1007/s10856-013-5018-y. Epub 2013 Aug 29. J Mater Sci Mater Med. 2013. PMID: 23990148
-
The dark side of soft tissues: Unexpected inorganic carbonate in the invasive slipper limpet Crepidula fornicata and its implications for stable isotope interpretations.Rapid Commun Mass Spectrom. 2019 Jan 15;33(1):107-115. doi: 10.1002/rcm.8322. Rapid Commun Mass Spectrom. 2019. PMID: 30376196
Cited by
-
Fabrication and Characterization of Polycaprolactone-Baghdadite Nanofibers by Electrospinning Method for Tissue Engineering Applications.Materials (Basel). 2024 Aug 23;17(17):4187. doi: 10.3390/ma17174187. Materials (Basel). 2024. PMID: 39274577 Free PMC article.
-
Ovine Mesenchymal Stem Cell Chondrogenesis on a Novel 3D-Printed Hybrid Scaffold In Vitro.Bioengineering (Basel). 2024 Jan 24;11(2):112. doi: 10.3390/bioengineering11020112. Bioengineering (Basel). 2024. PMID: 38391598 Free PMC article.
-
Poly-ε-Caprolactone 3D-Printed Porous Scaffold in a Femoral Condyle Defect Model Induces Early Osteo-Regeneration.Polymers (Basel). 2023 Dec 24;16(1):66. doi: 10.3390/polym16010066. Polymers (Basel). 2023. PMID: 38201731 Free PMC article.
-
Influence of Cell Seeding Density and Material Stiffness on Chondrogenesis of Human Stem Cells Within Soft Hydrogels, Without the Use of Exogenous Growth Factors.Gels. 2025 Mar 18;11(3):213. doi: 10.3390/gels11030213. Gels. 2025. PMID: 40136918 Free PMC article.
References
-
- Bohner M. Resorbable biomaterials as bone graft substitutes. Mater. Today. 2010;13:24–30. doi: 10.1016/S1369-7021(10)70014-6. - DOI
-
- Shibuya N., Jupiter D.C. Bone Graft Substitute: Allograft and Xenograft. [(accessed on 14 April 2023)];Clin. Podiatr. Med. Surg. 2015 32:21–34. doi: 10.1016/j.cpm.2014.09.011. Available online: https://www.sciencedirect.com/science/article/pii/S0891842214000846. - DOI - PubMed
-
- Fernandez de Grado G., Keller L., Idoux-Gillet Y., Wagner Q., Musset A.M., Benkirane-Jessel N., Bornert F., Offner D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018;9:2041731418776819. doi: 10.1177/2041731418776819. - DOI - PMC - PubMed
-
- Lin L., Chow K.L., Leng Y. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J. BioMed Mater. Res. Part A J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008;2:326–335. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

