Genus Acanthella-A Wealthy Treasure: Secondary Metabolites, Synthesis, Biosynthesis, and Bioactivities
- PMID: 37103397
- PMCID: PMC10141032
- DOI: 10.3390/md21040257
Genus Acanthella-A Wealthy Treasure: Secondary Metabolites, Synthesis, Biosynthesis, and Bioactivities
Abstract
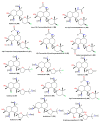
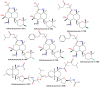
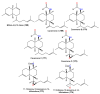
Marine sponges are multicellular and primitive animals that potentially represent a wealthy source of novel drugs. The genus Acanthella (family Axinellidae) is renowned to produce various metabolites with various structural characteristics and bioactivities, including nitrogen-containing terpenoids, alkaloids, and sterols. The current work provides an up-to-date literature survey and comprehensive insight into the reported metabolites from the members of this genus, as well as their sources, biosynthesis, syntheses, and biological activities whenever available. In the current work, 226 metabolites have been discussed based on published data from the period from 1974 to the beginning of 2023 with 90 references.
Keywords: Acanthella; Axinellidae; health and wellbeing; life below water; marine sponges; nitrogen-containing terpenoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures























Similar articles
-
Genus Smenospongia: Untapped Treasure of Biometabolites-Biosynthesis, Synthesis, and Bioactivities.Molecules. 2022 Sep 14;27(18):5969. doi: 10.3390/molecules27185969. Molecules. 2022. PMID: 36144705 Free PMC article. Review.
-
Spongia Sponges: Unabated Sources of Novel Secondary Metabolites.Mar Drugs. 2024 May 7;22(5):213. doi: 10.3390/md22050213. Mar Drugs. 2024. PMID: 38786604 Free PMC article. Review.
-
Natural Product Repertoire of the Genus Amphimedon.Mar Drugs. 2018 Dec 30;17(1):19. doi: 10.3390/md17010019. Mar Drugs. 2018. PMID: 30598005 Free PMC article. Review.
-
Genus Litophyton: A Hidden Treasure Trove of Structurally Unique and Diversely Bioactive Secondary Metabolites.Mar Drugs. 2023 Sep 29;21(10):523. doi: 10.3390/md21100523. Mar Drugs. 2023. PMID: 37888458 Free PMC article. Review.
-
Secondary Metabolites from the Marine Sponges of the Genus Petrosia: A Literature Review of 43 Years of Research.Mar Drugs. 2021 Feb 25;19(3):122. doi: 10.3390/md19030122. Mar Drugs. 2021. PMID: 33668842 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

