Pre-hospital freeze-dried plasma for critical bleeding after trauma: A pilot randomized controlled trial
- PMID: 37103482
- PMCID: PMC10946458
- DOI: 10.1111/acem.14745
Pre-hospital freeze-dried plasma for critical bleeding after trauma: A pilot randomized controlled trial
Abstract
Objectives: Transfusion of a high ratio of plasma to packed red blood cells (PRBCs), to treat or prevent acute traumatic coagulopathy, has been associated with survival after major trauma. However, the effect of prehospital plasma on patient outcomes has been inconsistent. The aim of this pilot trial was to assess the feasibility of transfusing freeze-dried plasma with red blood cells (RBCs) using a randomized controlled design in an Australian aeromedical prehospital setting.
Methods: Patients attended by helicopter emergency medical service (HEMS) paramedics with suspected critical bleeding after trauma managed with prehospital RBCs were randomized to receive 2 units of freeze-dried plasma (Lyoplas N-w) or standard care (no plasma). The primary outcome was the proportion of eligible patients enrolled and provided the intervention. Secondary outcomes included preliminary data on effectiveness, including mortality censored at 24 h and at hospital discharge, and adverse events.
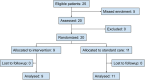
Results: During the study period of June 1 to October 31, 2022, there were 25 eligible patients, of whom 20 (80%) were enrolled in the trial and 19 (76%) received the allocated intervention. Median time from randomization to hospital arrival was 92.5 min (IQR 68-101.5 min). Mortality may have been lower in the freeze-dried plasma group at 24 h (RR 0.24, 95% CI 0.03-1.73) and at hospital discharge (RR 0.73, 95% CI 0.24-2.27). No serious adverse events related to the trial interventions were reported.
Conclusions: This first reported experience of freeze-dried plasma use in Australia suggests prehospital administration is feasible. Given longer prehospital times typically associated with HEMS attendance, there is potential clinical benefit from this intervention and rationale for a definitive trial.
© 2023 The Authors. Academic Emergency Medicine published by Wiley Periodicals LLC on behalf of Society for Academic Emergency Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3‐S11. - PubMed
-
- Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population‐based assessment. World J Surg. 2010;34:158‐163. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical