Coping After Breast Cancer: Protocol for a Randomized Controlled Trial of Stress Management eHealth Interventions
- PMID: 37103493
- PMCID: PMC10248777
- DOI: 10.2196/47195
Coping After Breast Cancer: Protocol for a Randomized Controlled Trial of Stress Management eHealth Interventions
Abstract
Background: One-third or more of breast cancer survivors report stress and other psychological and physical complaints that can negatively impact their quality of life. Psychosocial stress management interventions, shown to mitigate the negative impact of these complaints, can now be delivered as accessible and convenient (for the patient and provider) eHealth interventions. In this randomized controlled trial (RCT), Coping After Breast Cancer (CABC), 2 modified versions of the stress management eHealth intervention program StressProffen were created: one with predominantly cognitive behavioral stress management content (StressProffen-cognitive behavioral therapy intervention [StressProffen-CBI]) and another with predominantly mindfulness-based stress management content (StressProffen-mindfulness-based intervention [StressProffen-MBI]).
Objective: This study aims to investigate the effects in breast cancer survivors of using StressProffen-CBI and StressProffen-MBI compared with a control group (treatment as usual).
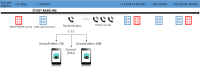
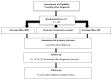
Methods: Women diagnosed with breast cancer (stage I-III, unequivocally human epidermal growth factor receptor 2-positive or estrogen receptor-negative tumors) or ductal carcinoma in situ (DCIS) aged 21-69 years who completed the Cancer Registry of Norway-initiated health survey on quality of life are invited to the CABC trial about 7 months after diagnosis. Women who give consent to participate are randomized (1:1:1) to either the StressProffen-CBI, StressProffen-MBI, or control group. Both StressProffen interventions consist of 10 modules of stress management content delivered through text, sound, video, and images. The primary outcome is between-group changes in perceived stress at 6 months, assessed with Cohen 10-item Perceived Stress Scale. The secondary outcomes comprise changes in quality of life, anxiety, depression, fatigue, sleep, neuropathy, coping, mindfulness, and work-related outcomes approximately 1, 2, and 3 years after diagnosis. Long-term effects of the interventions on work participation, comorbidities, relapse or new cancers, and mortality will be assessed using data from national health registries.
Results: Recruitment is scheduled from January 2021 to May 2023. The goal is to recruit 430 participants (100 in each group). As of April 14 2023, 428 participants have been enrolled.
Conclusions: The CABC trial is possibly the largest ongoing psychosocial eHealth RCT in patients with breast cancer. If 1 or both interventions prove to be effective in reducing stress and improving psychosocial and physical complains, the StressProffen eHealth interventions could be beneficial, inexpensive, and easily implementable tools for breast cancer survivors when coping with late effects after cancer and cancer treatments.
Trial registration: Clinicaltrials.gov NCT04480203; https://clinicaltrials.gov/ct2/show/NCT04480203.
International registered report identifier (irrid): DERR1-10.2196/47195.
Keywords: RCT; StressProffen; breast cancer; cognitive behavioral therapy; digital; eHealth; intervention; mindfulness; psychosocial; stress management.
©Karianne Svendsen, Lise Solberg Nes, Anders Meland, Ine Marie Larsson, Ylva M Gjelsvik, Elin Børøsund, Christine M Rygg, Tor Åge Myklebust, Kristin V Reinertsen, Cecilie E Kiserud, Helle Skjerven, Michael H Antoni, Trudie Chalder, Ingvil Mjaaland, Linda E Carlson, Hege R Eriksen, Giske Ursin. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 24.05.2023.
Conflict of interest statement
Conflicts of Interest: KS, AM, IML, EB, CMR, YMG, TÅM, HS, KVR, IM, CEK, and GU declare no conflicts of interest. LSN is an unpaid board member of dHealth AS, a company aiming to commercialize the original StressProffen program. TC is Co-chief investigator on a National Institute for Health and Care Research (NIHR) program grant in the UK testing a modified acceptance and commitment therapy intervention to improve quality of life for people with a range of cancers in the posttreatment phase. MHA is a consultant for Blue Note Therapeutics and Atlantis Healthcare, both digital health software companies. LEC receives royalties for 2 mindfulness book titles, and from eMindful for an online mindfulness-based cancer recovery program. HRE is a co-founder and a shareholder of a company offering lectures, education, and supervision about stress and coping (Stressprofessorene AS). HRE has no commercial interest in the StressProffen program.
Figures
Similar articles
-
Digital Cognitive Behavioral- and Mindfulness-Based Stress-Management Interventions for Survivors of Breast Cancer: Development Study.JMIR Form Res. 2023 Sep 19;7:e48719. doi: 10.2196/48719. JMIR Form Res. 2023. PMID: 37725424 Free PMC article.
-
Digital stress management in cancer: Testing StressProffen in a 12-month randomized controlled trial.Cancer. 2022 Apr 1;128(7):1503-1512. doi: 10.1002/cncr.34046. Epub 2021 Dec 2. Cancer. 2022. PMID: 34855212 Clinical Trial.
-
Results from a randomized controlled trial testing StressProffen; an application-based stress-management intervention for cancer survivors.Cancer Med. 2020 Jun;9(11):3775-3785. doi: 10.1002/cam4.3000. Epub 2020 Apr 3. Cancer Med. 2020. PMID: 32243717 Free PMC article. Clinical Trial.
-
eHealth Interventions for Dutch Cancer Care: Systematic Review Using the Triple Aim Lens.JMIR Cancer. 2022 Jun 14;8(2):e37093. doi: 10.2196/37093. JMIR Cancer. 2022. PMID: 35699991 Free PMC article. Review.
-
Long-Term Fatigue and Cognitive Disorders in Breast Cancer Survivors.Cancers (Basel). 2019 Nov 28;11(12):1896. doi: 10.3390/cancers11121896. Cancers (Basel). 2019. PMID: 31795208 Free PMC article. Review.
Cited by
-
How Did Breast Cancer Patients Fare during Different Phases of the COVID-19 Pandemic in Norway Compared to Age-Matched Controls?Cancers (Basel). 2024 Jan 31;16(3):602. doi: 10.3390/cancers16030602. Cancers (Basel). 2024. PMID: 38339359 Free PMC article.
-
Digital Cognitive Behavioral- and Mindfulness-Based Stress-Management Interventions for Survivors of Breast Cancer: Development Study.JMIR Form Res. 2023 Sep 19;7:e48719. doi: 10.2196/48719. JMIR Form Res. 2023. PMID: 37725424 Free PMC article.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7–33. doi: 10.3322/caac.21708. https://onlinelibrary.wiley.com/doi/10.3322/caac.21708 - DOI - DOI - PubMed
-
- The Cancer Registry of Norway . The Cancer Registry of Norway. Oslo, Norway: The Cancer Registry of Norway; 2022. [2023-08-04]. Cancer incidence, mortality, survival and prevalence in Norway 2021. https://www.kreftregisteret.no/globalassets/cancer-in-norway/2021/cin_re... .
-
- Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol. 1994 May;49(5):389–404. doi: 10.1037//0003-066x.49.5.389. https://europepmc.org/abstract/MED/8024167 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

