Long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension
- PMID: 37103755
- PMCID: PMC10134722
- DOI: 10.1007/s00415-023-11699-x
Long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension
Abstract
Introduction: Ravulizumab demonstrated efficacy and an acceptable safety profile versus placebo in the randomized controlled period (RCP) of the phase 3 CHAMPION MG trial in patients with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis. We report an interim analysis of the ongoing open-label extension (OLE) designed to evaluate long-term treatment effects.
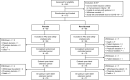
Methods: Following completion of the 26-week RCP, patients could enter the OLE; patients who received ravulizumab in the RCP continued the drug; patients who previously received placebo switched to ravulizumab. Patients receive body-weight-based maintenance dosing of ravulizumab every 8 weeks. Efficacy endpoints up to 60 weeks included Myasthenia Gravis-Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) scores, with least-squares (LS) mean change and 95% confidence intervals (95% CI) reported.
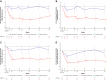
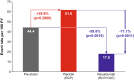
Results: Long-term efficacy and safety in the OLE were analyzed in 161 and 169 patients, respectively. Improvements in all scores were maintained through 60 weeks in patients who received ravulizumab during the RCP; LS mean change from RCP baseline in MG-ADL score was - 4.0 (95% CI: - 4.8, - 3.1; p < 0.0001). Rapid (within 2 weeks) and sustained improvements occurred in patients previously receiving placebo; LS mean change in MG-ADL score from OLE baseline to Week 60 was - 1.7 (95% CI: - 2.7, - 0.8; p = 0.0007). Similar trends were seen in QMG scores. Ravulizumab treatment was associated with a decreased rate of clinical deterioration events compared with placebo. Ravulizumab was well tolerated; no meningococcal infections were reported.
Conclusion: Findings support the sustained efficacy and long-term safety of ravulizumab, administered every 8 weeks, in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis.
Clinicaltrials: gov identifier: NCT03920293; EudraCT: 2018-003243-39.
Keywords: Generalized myasthenia gravis; Long-term; Open-label; Ravulizumab.
© 2023. The Author(s).
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

