Redox mechanisms and their pathological role in prion diseases: The road to ruin
- PMID: 37104170
- PMCID: PMC10138251
- DOI: 10.1371/journal.ppat.1011309
Redox mechanisms and their pathological role in prion diseases: The road to ruin
Abstract
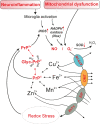
Prion diseases, also known as transmissible spongiform encephalopathies, are rare, progressive, and fatal neurodegenerative disorders, which are caused by the accumulation of the misfolded cellular prion protein (PrPC). The resulting cytotoxic prion species, referred to as the scrapie prion isoform (PrPSc), assemble in aggregates and interfere with neuronal pathways, ultimately rendering neurons dysfunctional. As the prion protein physiologically interacts with redox-active metals, an altered redox balance within the cell can impact these interactions, which may lead to and facilitate further misfolding and aggregation. The initiation of misfolding and the aggregation processes will, in turn, induce microglial activation and neuroinflammation, which leads to an imbalance in cellular redox homeostasis and enhanced redox stress. Potential approaches for therapeutics target redox signalling, and this review illustrates the pathways involved in the above processes.
Copyright: © 2023 Spiers et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Neuropathology of Animal Prion Diseases.Biomolecules. 2021 Mar 21;11(3):466. doi: 10.3390/biom11030466. Biomolecules. 2021. PMID: 33801117 Free PMC article. Review.
-
Prion Diseases.Adv Neurobiol. 2017;15:335-364. doi: 10.1007/978-3-319-57193-5_13. Adv Neurobiol. 2017. PMID: 28674988 Review.
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
-
Prion peptide 106-126 as a model for prion replication and neurotoxicity.Front Biosci. 2002 Apr 1;7:a60-71. doi: 10.2741/A740. Front Biosci. 2002. PMID: 11897566
-
FTIR-microspectroscopy of prion-infected nervous tissue.Biochim Biophys Acta. 2006 Jul;1758(7):948-59. doi: 10.1016/j.bbamem.2006.05.026. Epub 2006 Jun 7. Biochim Biophys Acta. 2006. PMID: 16887095 Review.
References
-
- Carroll JA, Striebel JF, Race B, Phillips K, Chesebro B. Prion infection of mouse brain reveals multiple new upregulated genes involved in neuroinflammation or signal transduction. J Virol. 2015;89(4):2388–2404. Epub 20141210. doi: 10.1128/JVI.02952-14 ; PubMed Central PMCID: PMC4338885. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

