Anti-Metalloproteases: Production and Characterization of Polyclonal IgG Anti-F2 Fraction Antibodies Purified from the Venom of the Snake Bitis arietans
- PMID: 37104202
- PMCID: PMC10145261
- DOI: 10.3390/toxins15040264
Anti-Metalloproteases: Production and Characterization of Polyclonal IgG Anti-F2 Fraction Antibodies Purified from the Venom of the Snake Bitis arietans
Abstract
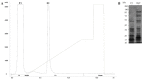
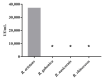
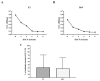
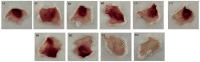
Bitis arietans is a medically important snake found in Sub-Saharan Africa. The envenomation is characterized by local and systemic effects, and the lack of antivenoms aggravates the treatment. This study aimed to identify venom toxins and develop antitoxins. The F2 fraction obtained from Bitis arietans venom (BaV) demonstrated the presence of several proteins in its composition, including metalloproteases. Titration assays carried out together with the immunization of mice demonstrated the development of anti-F2 fraction antibodies by the animals. The determination of the affinity of antibodies against different Bitis venoms was evaluated, revealing that only BaV had peptides recognized by anti-F2 fraction antibodies. In vivo analyses demonstrated the hemorrhagic capacity of the venom and the effectiveness of the antibodies in inhibiting up to 80% of the hemorrhage and 0% of the lethality caused by BaV. Together, the data indicate: (1) the prevalence of proteins that influence hemostasis and envenomation; (2) the effectiveness of antibodies in inhibiting specific activities of BaV; and (3) isolation and characterization of toxins can become crucial steps in the development of new alternative treatments. Thus, the results obtained help in understanding the envenoming mechanism and may be useful for the study of new complementary therapies.
Keywords: Bitis arietans; antivenoms; metalloproteases; polyclonal antibodies.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kasturiratne A., Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lalloo D.G., de Silva H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:1591–1604. doi: 10.1371/journal.pmed.0050218. - DOI - PMC - PubMed
-
- WHO . Guidelines for the Prevention and Clinical Management of Snakebite in Africa. World Health Organization; Brazzaville, Congo: 2010. pp. 1–145.
-
- Lenk P., Herrmann H.W., Joger U., Wink M. Phylogeny and taxonomic subdivision of Bitis (Reptilia: Viperidae) based on molecular evidence. Kaupia. 1999;8:31–38.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

