An Assassin's Secret: Multifunctional Cytotoxic Compounds in the Predation Venom of the Assassin Bug Psytalla horrida (Reduviidae, Hemiptera)
- PMID: 37104240
- PMCID: PMC10144120
- DOI: 10.3390/toxins15040302
An Assassin's Secret: Multifunctional Cytotoxic Compounds in the Predation Venom of the Assassin Bug Psytalla horrida (Reduviidae, Hemiptera)
Abstract
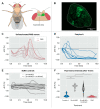
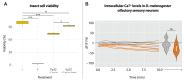
Predatory assassin bugs produce venomous saliva that enables them to overwhelm, kill, and pre-digest large prey animals. Venom from the posterior main gland (PMG) of the African assassin bug Psytalla horrida has strong cytotoxic effects, but the responsible compounds are yet unknown. Using cation-exchange chromatography, we fractionated PMG extracts from P. horrida and screened the fractions for toxicity. Two venom fractions strongly affected insect cell viability, bacterial growth, erythrocyte integrity, and intracellular calcium levels in Drosophila melanogaster olfactory sensory neurons. LC-MS/MS analysis revealed that both fractions contained gelsolin, redulysins, S1 family peptidases, and proteins from the uncharacterized venom protein family 2. Synthetic peptides representing the putative lytic domain of redulysins had strong antimicrobial activity against Escherichia coli and/or Bacillus subtilis but only weak toxicity towards insect or mammalian cells, indicating a primary role in preventing the intake of microbial pathogens. In contrast, a recombinant venom protein family 2 protein significantly reduced insect cell viability but exhibited no antibacterial or hemolytic activity, suggesting that it plays a role in prey overwhelming and killing. The results of our study show that P. horrida secretes multiple cytotoxic compounds targeting different organisms to facilitate predation and antimicrobial defense.
Keywords: Reduviidae; cytotoxicity; redulysin; venom protein family 2; venomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

