Dose-Escalated Radiotherapy Alone or in Combination With Short-Term Androgen Deprivation for Intermediate-Risk Prostate Cancer: Results of a Phase III Multi-Institutional Trial
- PMID: 37104748
- PMCID: PMC10489479
- DOI: 10.1200/JCO.22.02390
Dose-Escalated Radiotherapy Alone or in Combination With Short-Term Androgen Deprivation for Intermediate-Risk Prostate Cancer: Results of a Phase III Multi-Institutional Trial
Abstract
Purpose: It remains unknown whether or not short-term androgen deprivation (STAD) improves survival among men with intermediate-risk prostate cancer (IRPC) treated with dose-escalated radiotherapy (RT).
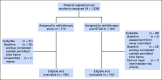
Methods: The NRG Oncology/Radiation Therapy Oncology Group 0815 study randomly assigned 1,492 patients with stage T2b-T2c, Gleason score 7, or prostate-specific antigen (PSA) value >10 and ≤20 ng/mL to dose-escalated RT alone (arm 1) or with STAD (arm 2). STAD was 6 months of luteinizing hormone-releasing hormone agonist/antagonist therapy plus antiandrogen. RT modalities were external-beam RT alone to 79.2 Gy or external beam (45 Gy) with brachytherapy boost. The primary end point was overall survival (OS). Secondary end points included prostate cancer-specific mortality (PCSM), non-PCSM, distant metastases (DMs), PSA failure, and rates of salvage therapy.
Results: Median follow-up was 6.3 years. Two hundred nineteen deaths occurred, 119 in arm 1 and 100 in arm 2. Five-year OS estimates were 90% versus 91%, respectively (hazard ratio [HR], 0.85; 95% CI, 0.65 to 1.11]; P = .22). STAD resulted in reduced PSA failure (HR, 0.52; P <.001), DM (HR, 0.25; P <.001), PCSM (HR, 0.10; P = .007), and salvage therapy use (HR, 0.62; P = .025). Other-cause deaths were not significantly different (P = .56). Acute grade ≥3 adverse events (AEs) occurred in 2% of patients in arm 1 and in 12% for arm 2 (P <.001). Cumulative incidence of late grade ≥3 AEs was 14% in arm 1 and 15% in arm 2 (P = .29).
Conclusion: STAD did not improve OS rates for men with IRPC treated with dose-escalated RT. Improvements in metastases rates, prostate cancer deaths, and PSA failures should be weighed against the risk of adverse events and the impact of STAD on quality of life.
Trial registration: ClinicalTrials.gov NCT00936390.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


Comment in
-
Re: Dose-escalated Radiotherapy Alone or in Combination with Short-term Androgen Deprivation for Intermediate-risk Prostate Cancer: Results of a Phase III Multi-institutional Trial.Eur Urol. 2023 Dec;84(6):600-601. doi: 10.1016/j.eururo.2023.06.030. Epub 2023 Jul 11. Eur Urol. 2023. PMID: 37438199 No abstract available.
References
-
- Gandaglia G, Leni R, Bray F, et al. : Epidemiology and prevention of prostate cancer. Eur Urol Oncol 4:877-892, 2021 - PubMed
-
- Jones CU, Hunt D, McGowan DG, et al. : Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 365:107-118, 2011 - PubMed
-
- Pilepich MV, Winter K, John MJ, et al. : Phase III Radiation Therapy Oncology Group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys 50:1243-1252, 2001 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

