Distinct and joint effects of low and high levels of Aβ and tau deposition on cortical thickness
- PMID: 37104927
- PMCID: PMC10165160
- DOI: 10.1016/j.nicl.2023.103409
Distinct and joint effects of low and high levels of Aβ and tau deposition on cortical thickness
Abstract
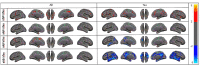
Alzheimer's disease (AD) is defined by the presence of Amyloid-β (Aβ),tau, and neurodegeneration (ATN framework) in the human cerebral cortex. Yet, prior studies have suggested that Aβ deposition can be associated with both cortical thinning and thickening. These contradictory results are attributed to small sample sizes, the presence versus absence of tau, and limited detectability in the earliest phase of protein deposition, which may begin in young adulthood and cannot be captured in studies enrolling only older subjects. In this study, we aimed to find the distinct and joint effects of Aβ andtau on neurodegeneration during the progression from normal to abnormal stages of pathologies that remain elusive. We used18F-MK6240 and 18F-Florbetaben/18F-Florbetapir positron emission tomography (PET) and magnetic resonance imaging (MRI) to quantify tau, Aβ, and cortical thickness in 590 participants ranging in age from 20 to 90. We performed multiple regression analyses to assess the distinct and joint effects of Aβ and tau on cortical thickness using 590 healthy control (HC) and mild cognitive impairment (MCI) participants (141 young, 394 HC elderlies, 52 MCI). We showed thatin participants with normal levels of global Aβdeposition, Aβ uptakewassignificantly associated with increasedcortical thickness regardless of tau (e.g., left entorhinal cortex with t > 3.241, p < 0.0013). The relationship between tau deposition and neurodegeneration was more complex: in participants with abnormal levels of global tau, tau uptake was associated with cortical thinning in several regions of the brain (e.g., left entorhinal with t < -2.80, p < 0.0096 and left insula with t-value < -4.284, p < 0.0001), as reported on prior neuroimaging and neuropathological studies. Surprisingly, in participants with normal levels of global tau, tau was found to be associated with cortical thickening. Moreover, in participants with abnormal levels of global Aβandtau, theresonancebetween them, defined as their correlation throughout the cortex, wasassociated strongly with cortical thinning even when controlling for a direct linear effect. We confirm prior findings of an association between Aβ deposition and cortical thickening and suggest this may also be the case in the earliest stages of deposition in normal aging. We also illustrate that resonance between high levels of Aβ and tau uptake is strongly associated with cortical thinning, emphasizing the effects of Aβ/tau synergy inAD pathogenesis.
Keywords: Alzheimer's disease; Amyloid-β; Cortical thickness; Neurodegeneration; Tau.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Ashrafian H., et al. Review on Alzheimer's disease: inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021;167:382–394. - PubMed
-
- Avants B.B., et al. Advanced normalization tools (ANTS) Insight j. 2009;2:1–35.
-
- Batzu L., et al. Cerebrospinal fluid progranulin is associated with increased cortical thickness in early stages of Alzheimer's disease. Neurobiol. Aging. 2020;88:61–70. - PubMed
-
- Becker J., et al. Amyloid deposition and brain volume across the continuum of aging and AD. Ann Neurol. 2011
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

