Circulating tumor DNA analysis of the phase III VOYAGER trial: KIT mutational landscape and outcomes in patients with advanced gastrointestinal stromal tumor treated with avapritinib or regorafenib
- PMID: 37105265
- PMCID: PMC10330293
- DOI: 10.1016/j.annonc.2023.04.006
Circulating tumor DNA analysis of the phase III VOYAGER trial: KIT mutational landscape and outcomes in patients with advanced gastrointestinal stromal tumor treated with avapritinib or regorafenib
Abstract
Background: The current treatment paradigm of imatinib-resistant metastatic gastrointestinal stromal tumor (GIST) does not incorporate KIT/PDGFRA genotypes in therapeutic drug sequencing, except for PDGFRA exon 18-mutant GIST that is indicated for avapritinib treatment. Here, circulating tumor DNA (ctDNA) sequencing was used to analyze plasma samples prospectively collected in the phase III VOYAGER trial to understand how the KIT/PDGFRA mutational landscape contributes to tyrosine kinase inhibitor (TKI) resistance and to determine its clinical validity and utility.
Patients and methods: VOYAGER (N = 476) compared avapritinib with regorafenib in patients with KIT/PDGFRA-mutant GIST previously treated with imatinib and one or two additional TKIs (NCT03465722). KIT/PDGFRA ctDNA mutation profiling of plasma samples at baseline and end of treatment was assessed with 74-gene Guardant360® CDx. Molecular subgroups were determined and correlated with outcomes.
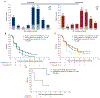
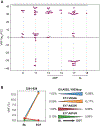
Results: A total of 386/476 patients with KIT/PDGFRA-mutant tumors underwent baseline (pre-trial treatment) ctDNA analysis; 196 received avapritinib and 190 received regorafenib. KIT and PDGFRA mutations were detected in 75.1% and 5.4%, respectively. KIT resistance mutations were found in the activation loop (A-loop; 80.4%) and ATP-binding pocket (ATP-BP; 40.8%); 23.4% had both. An average of 2.6 KIT mutations were detected per patient; 17.2% showed 4-14 different KIT resistance mutations. Of all pathogenic KIT variants, 28.0% were novel, including alterations in exons/codons previously unreported. PDGFRA mutations showed similar patterns. ctDNA-detected KIT ATP-BP mutations negatively prognosticated avapritinib activity, with a median progression-free survival (mPFS) of 1.9 versus 5.6 months for regorafenib. mPFS for regorafenib did not vary regardless of the presence or absence of ATP-BP/A-loop mutants and was greater than mPFS with avapritinib in this population. Secondary KIT ATP-BP pocket mutation variants, particularly V654A, were enriched upon disease progression with avapritinib.
Conclusions: ctDNA sequencing efficiently detects KIT/PDGFRA mutations and prognosticates outcomes in patients with TKI-resistant GIST treated with avapritinib. ctDNA analysis can be used to monitor disease progression and provide more personalized treatment.
Keywords: GIST; KIT; PDGFRA; avapritinib; ctDNA; regorafenib.
Copyright © 2023 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
CS has received research funding (institution) from IDRX, Blueprint, Karyopharm, Pfizer, Deciphera, and Bayer; consulting fees (advisory role) from IDRX, CogentBio, Immunicum AB, Deciphera and Blueprint; payment for lectures from Deciphera, PharmaMar, Pfizer, Bayer and Blueprint; and travel grants from PharmaMar, Gilead, Pfizer, and Bayer.
SBa has received honoraria from Novartis, Pfizer, Bayer, Pharmamar, and GlaxoSmithKline; reports consulting or advisory role for Blueprint Medicines Corporation, Bayer, Lilly, Deciphera, Nanobiotix, Daiichi Sankyo, Exelixis, Janssen-Cilag, ADC Therapeutics, Mundipharma, and GlaxoSmithKline; research funding from Blueprint Medicines Corporation, Novartis, and Incyte; travel and accommodation expenses from Pharmamar.
DG-P declares no conflicts of interest.
Y-K K reports consulting or advisory role for DAEHWA Pharmaceutical, Bristol Myers Squibb, Zymeworks, ALX Oncology, Amgen, Novartis, Macrogenics, Surface Oncology, and Blueprint Medicines Corporation.
RLJ reports consulting or advisory role for Lilly, Immune Design, Merck Serono, Adaptimmune, Daiichi Sankyo, Eisai, Morphotek, TRACON Pharma, Immodulon Therapeutics, Deciphera, PharmaMar, Blueprint Medicines Corporation, Clinigen Group, Epizyme, Boehringer Ingelheim, Bayer, Karma Oncology, and UpToDate; research funding from GlaxoSmithKline; travel and accommodation, expenses from PharmaMar.
PR has received honoraria from Bristol Myers Squibb, MSD, Novartis, Roche, Lilly, Pfizer, Pierre Fabre, Sanofi, and Merck; reports consulting or advisory role for Novartis, Blueprint Medicines Corporation, and Bristol Myers Squibb.
OM is an employee and shareholder of Amgen, Inc.
MCH reports stock and other ownership interests in MolecularMD; honoraria from Novartis; consulting or advisory role for MolecularMD, Novartis, Blueprint Medicines Corporation, Deciphera, C Stone Pharmaceuticals, Zai Labs, and Theseus Pharmaceuticals; patents, royalties, and other intellectual property regarding patent on treatment of GIST-licensed to Novartis; expert testimony for Novartis.
WDT has a leadership role in Certis Oncology Solutions, Atropos, and Innova Therapeutics; reports owning stock and other ownership interests in Certis Oncology Solutions, and Atropos; consulting or advisory role for EMD Serono, Lilly, Daiichi Sankyo, Blueprint Medicines Corporation, Agios, NanoCarrier, Deciphera, C4 Therapeutics, Mundipharma, Adcendo, Ayala Pharmaceuticals, Kowa Pharmaceutical, Servier, and AbMaxBio; research funding from Novartis, Lilly, Plexxikon, Daiichi Sankyo, TRACON Pharma, Blueprint Medicines Corporation, Immune Design, BioAtla, and Deciphera; patents, royalties, and other intellectual property regarding Companion Diagnostic for CDK4 inhibitors—14/854,329, Enigma and CDH18 as companion Diagnostics for CDK4 inhibition—SKI2016-021-03
KN is an employee and equity holder of Blueprint Medicines Corporation.
AG is an employee and equity holder of Blueprint Medicines Corporation.
HS is an employee and equity holder of Blueprint Medicines Corporation.
SBi reports no conflicts of interest.
PS has received honoraria from Deciphera, Blueprint Medicines Corporation, Boehringer and Ingelheim; consulting or advisory role for Blueprint Medicines Corporation, Ellipses Pharma, Adaptimmune, Intellisphere, Transgene, Deciphera, Exelixis, Boehringer Ingelheim, Medscape, Guided Clarity, Ysios Capital, and Studiecentrum voor Kernenergie;research funding from CoBioRes NV, Eisai, G1 Therapeutics, Novartis, and PharmaMar; travel and accommodations expenses from MSD, Ipsen, and Boehringer Ingelheim.
MAP has received research funding (institution) from Novartis; consulting fees (advisory role) from Roche, PharmaMar; lecture fee from Pfizer, Eli Lilly.
MvM reports consulting or advisory role for Deciphera, and Exelixis; research funding from ArQule, Novartis, Blueprint Medicines Corporation, Deciphera, Gradalis, Springworks Therapeutics, Lilly, Arog, Genmab, and ASCO; travel and accommodations expenses from Deciphera Pharmaceuticals, and NCCN.
JCT reports consulting or advisory role for Novartis, Lilly, Janssen, and Blueprint Medicines Corporation, Deciphera, Daiichi Sankyo, Epizyme, Agios, C4 Therapeutics, AADI, and Bayer.
SG reports owning stock and other ownership interests in Abbott Laboratories; consulting or advisory role for Blueprint Medicines Corporation, Deciphera, Bayer, Lilly, UpToDate, Research to Practice, MORE Health, Daiichi, and Kayothera; research funding from Blueprint Medicines Corporation, Deciphera, Daiichi Sankyo RD Novare, Merck, Eisai, SpringWorks Therapeutics, Theseus, and IDRX; patents, royalties, and other intellectual property reported for UptoDateExpert; testimony for Bayer; other relationship reported for Research to Practice, and WCG.
Figures

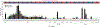

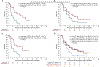
Similar articles
-
Avapritinib Versus Regorafenib in Locally Advanced Unresectable or Metastatic GI Stromal Tumor: A Randomized, Open-Label Phase III Study.J Clin Oncol. 2021 Oct 1;39(28):3128-3139. doi: 10.1200/JCO.21.00217. Epub 2021 Aug 3. J Clin Oncol. 2021. PMID: 34343033 Free PMC article. Clinical Trial.
-
[The importance of mutational status in prognosis and therapy of GIST].Recenti Prog Med. 2015 Jan;106(1):17-22. doi: 10.1701/1740.18950. Recenti Prog Med. 2015. PMID: 25621775 Italian.
-
Robust Activity of Avapritinib, Potent and Highly Selective Inhibitor of Mutated KIT, in Patient-derived Xenograft Models of Gastrointestinal Stromal Tumors.Clin Cancer Res. 2019 Jan 15;25(2):609-618. doi: 10.1158/1078-0432.CCR-18-1858. Epub 2018 Oct 1. Clin Cancer Res. 2019. PMID: 30274985
-
Correlation of imatinib resistance with the mutational status of KIT and PDGFRA genes in gastrointestinal stromal tumors: a meta-analysis.J Gastrointestin Liver Dis. 2013 Dec;22(4):413-8. J Gastrointestin Liver Dis. 2013. PMID: 24369323 Review.
-
Avapritinib in the treatment of PDGFRA exon 18 mutated gastrointestinal stromal tumors.Future Oncol. 2020 Aug;16(22):1639-1646. doi: 10.2217/fon-2020-0348. Epub 2020 Jun 10. Future Oncol. 2020. PMID: 32517495 Review.
Cited by
-
Characteristics and therapeutic strategies for familial gastrointestinal stromal tumors.World J Gastrointest Oncol. 2025 Mar 15;17(3):100463. doi: 10.4251/wjgo.v17.i3.100463. World J Gastrointest Oncol. 2025. PMID: 40092960 Free PMC article.
-
The potential of lenvatinib in breast cancer therapy.Med Oncol. 2024 Aug 22;41(9):233. doi: 10.1007/s12032-024-02477-4. Med Oncol. 2024. PMID: 39172293 Review.
-
Molecular Advances in the Treatment of Advanced Gastrointestinal Stromal Tumor.Oncologist. 2023 Aug 3;28(8):671-681. doi: 10.1093/oncolo/oyad167. Oncologist. 2023. PMID: 37315115 Free PMC article. Review.
-
Gastrointestinal stromal tumor mimicking perineurioma: A case report.Cytojournal. 2024 Nov 23;21:51. doi: 10.25259/Cytojournal_104_2024. eCollection 2024. Cytojournal. 2024. PMID: 39737118 Free PMC article.
-
Interrupted Systemic Therapy (Drug Holiday) for Metastatic Sarcoma: Is It Safe?Curr Treat Options Oncol. 2025 Jul;26(7):648-653. doi: 10.1007/s11864-025-01338-0. Epub 2025 Jun 27. Curr Treat Options Oncol. 2025. PMID: 40576756 Review.
References
-
- Heitzer E, Haque IS, Roberts CES, et al. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20(2):71–88. - PubMed
-
- Serrano C, George S. Gastrointestinal stromal tumor: challenges and opportunities for a new decade. Clin Cancer Res 2020;26(19):5078–5085. - PubMed
-
- Gómez-Peregrina D, García-Valverde A, Pilco-Janeta D, et al. Liquid biopsy in gastrointestinal stromal tumors: ready for prime time? Curr Treat Options Oncol. 2021;22(4):32. - PubMed
-
- Arshad J, Roberts A, Ahmed J, et al. Utility of circulating tumor DNA in the management of patients with GI stromal tumor: analysis of 243 patients. JCO Precis Oncol. 2020;4:66–73. - PubMed
-
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-KIT in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

