BICD2 phosphorylation regulates dynein function and centrosome separation in G2 and M
- PMID: 37105961
- PMCID: PMC10140047
- DOI: 10.1038/s41467-023-38116-1
BICD2 phosphorylation regulates dynein function and centrosome separation in G2 and M
Abstract
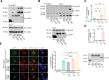
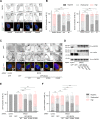
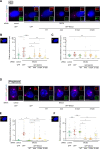
The activity of dynein is regulated by a number of adaptors that mediate its interaction with dynactin, effectively activating the motor complex while also connecting it to different cargos. The regulation of adaptors is consequently central to dynein physiology but remains largely unexplored. We now describe that one of the best-known dynein adaptors, BICD2, is effectively activated through phosphorylation. In G2, phosphorylation of BICD2 by CDK1 promotes its interaction with PLK1. In turn, PLK1 phosphorylation of a single residue in the N-terminus of BICD2 results in a structural change that facilitates the interaction with dynein and dynactin, allowing the formation of active motor complexes. Moreover, modified BICD2 preferentially interacts with the nucleoporin RanBP2 once RanBP2 has been phosphorylated by CDK1. BICD2 phosphorylation is central for dynein recruitment to the nuclear envelope, centrosome tethering to the nucleus and centrosome separation in the G2 and M phases of the cell cycle. This work reveals adaptor activation through phosphorylation as crucial for the spatiotemporal regulation of dynein activity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures