Structural and functional analysis of the newt lymphatic system
- PMID: 37106059
- PMCID: PMC10140069
- DOI: 10.1038/s41598-023-34169-w
Structural and functional analysis of the newt lymphatic system
Abstract
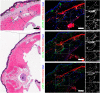
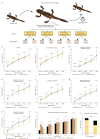
Regeneration competent vertebrates such as newts and salamanders possess a weakened adaptive immune system characterized by multiple connections between the lymphatic system and the blood vascular system called lymphatic hearts. The role of lymphatic vasculature and these lymphaticovenous connections in regeneration is unknown. We used in-vivo near-infrared lymphangiography, ultra-high frequency ultrasonography, micro-CT lymphangiography, and histological serial section 3-dimentional computer reconstruction to evaluate the lymphatic territories of Cynops pyrrhogaster. We used our model and supermicrosurgery to show that lymphatic hearts are not essential for lymphatic circulation and limb regeneration. Instead, newts possess a novel intraosseous network of lymphatics inside the bone expressing VEGFR-3, LYVE-1 and CD-31. However, we were unable to show Prox-1 expression by these vessels. We demonstrate that adult newt bone marrow functions as both a lymphatic drainage organ and fat reservoir. This study reveals the fundamental anatomical differences between the immune system of urodeles and mammals and provides a model for investigating lymphatics and regeneration.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

