1H magnetic resonance spectroscopic imaging of deuterated glucose and of neurotransmitter metabolism at 7 T in the human brain
- PMID: 37106154
- PMCID: PMC10861140
- DOI: 10.1038/s41551-023-01035-z
1H magnetic resonance spectroscopic imaging of deuterated glucose and of neurotransmitter metabolism at 7 T in the human brain
Abstract
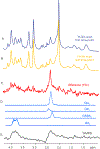
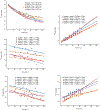
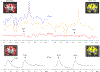
Impaired glucose metabolism in the brain has been linked to several neurological disorders. Positron emission tomography and carbon-13 magnetic resonance spectroscopic imaging (MRSI) can be used to quantify the metabolism of glucose, but these methods involve exposure to radiation, cannot quantify downstream metabolism, or have poor spatial resolution. Deuterium MRSI (2H-MRSI) is a non-invasive and safe alternative for the quantification of the metabolism of 2H-labelled substrates such as glucose and their downstream metabolic products, yet it can only measure a limited number of deuterated compounds and requires specialized hardware. Here we show that proton MRSI (1H-MRSI) at 7 T has higher sensitivity, chemical specificity and spatiotemporal resolution than 2H-MRSI. We used 1H-MRSI in five volunteers to differentiate glutamate, glutamine, γ-aminobutyric acid and glucose deuterated at specific molecular positions, and to simultaneously map deuterated and non-deuterated metabolites. 1H-MRSI, which is amenable to clinically available magnetic-resonance hardware, may facilitate the study of glucose metabolism in the brain and its potential roles in neurological disorders.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
R. Lanzenberger received travel grants and/or conference speaker honoraria within the last three years from Bruker BioSpin MR andHeel, and has served as a consultant for Ono Pharmaceutical. He received investigator-initiated research funding from Siemens Healthcare regarding clinical research using PET/MR. He is a shareholder of the start-up company BM Health GmbH since 2019.
The other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

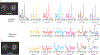

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

