Optimization of polymyxin B regimens for the treatment of carbapenem-resistant organism nosocomial pneumonia: a real-world prospective study
- PMID: 37106370
- PMCID: PMC10142183
- DOI: 10.1186/s13054-023-04448-z
Optimization of polymyxin B regimens for the treatment of carbapenem-resistant organism nosocomial pneumonia: a real-world prospective study
Abstract
Background: Polymyxin B is the first-line therapy for Carbapenem-resistant organism (CRO) nosocomial pneumonia. However, clinical data for its pharmacokinetic/pharmacodynamic (PK/PD) relationship are limited. This study aimed to investigate the relationship between polymyxin B exposure and efficacy for the treatment of CRO pneumonia in critically ill patients, and to optimize the individual dosing regimens.
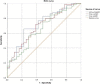
Methods: Patients treated with polymyxin B for CRO pneumonia were enrolled. Blood samples were assayed using a validated high-performance liquid chromatography-tandem mass spectrometry method. Population PK analysis and Monte Carlo simulation were performed using Phoenix NLME software. Logistic regression analyses and receiver operating characteristic (ROC) curve were employed to identify the significant predictors and PK/PD indices of polymyxin B efficacy.
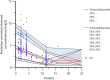
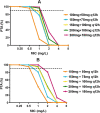
Results: A total of 105 patients were included, and the population PK model was developed based on 295 plasma concentrations. AUCss,24 h/MIC (AOR = 0.97, 95% CI 0.95-0.99, p = 0.009), daily dose (AOR = 0.98, 95% CI 0.97-0.99, p = 0.028), and combination of inhaled polymyxin B (AOR = 0.32, 95% CI 0.11-0.94, p = 0.039) were independent risk factors for polymyxin B efficacy. ROC curve showed that AUCss,24 h/MIC is the most predictive PK/PD index of polymyxin B for the treatment of nosocomial pneumonia caused by CRO, and the optimal cutoff point value was 66.9 in patients receiving combination therapy with another antimicrobial. Model-based simulation suggests that the maintaining daily dose of 75 and 100 mg Q12 h could achieve ≥ 90% PTA of this clinical target at MIC values ≤ 0.5 and 1 mg/L, respectively. For patients unable to achieve the target concentration by intravenous administration, adjunctive inhalation of polymyxin B would be beneficial.
Conclusions: For CRO pneumonia, daily dose of 75 and 100 mg Q12 h was recommended for clinical efficacy. Inhalation of polymyxin B is beneficial for patients who cannot achieve the target concentration by intravenous administration.
Keywords: Carbapenem-resistant organism; Dosing optimization; Nosocomial pneumonia; Pharmacokinetic/pharmacodynamic; Polymyxin B.
© 2023. The Author(s).
Conflict of interest statement
All authors report no competing interests.
Figures


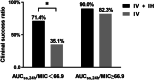
Similar articles
-
Pharmacokinetics and pharmacodynamics of polymyxin B and proposed dosing regimens in elderly patients with multi-drug-resistant Gram-negative bacterial infections.Int J Antimicrob Agents. 2022 Nov-Dec;60(5-6):106693. doi: 10.1016/j.ijantimicag.2022.106693. Epub 2022 Nov 11. Int J Antimicrob Agents. 2022. PMID: 36375775
-
Monte Carlo simulation to optimize polymyxin B dosing regimens for the treatment of Gram-negative bacteremia.Front Cell Infect Microbiol. 2025 Feb 26;15:1533177. doi: 10.3389/fcimb.2025.1533177. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40078873 Free PMC article.
-
Population pharmacokinetics of polymyxin B in critically ill patients receiving continuous venovenous haemofiltration.Int J Antimicrob Agents. 2022 Jul;60(1):106599. doi: 10.1016/j.ijantimicag.2022.106599. Epub 2022 May 5. Int J Antimicrob Agents. 2022. PMID: 35526750
-
Optimizing antibiotic dosing regimens for nosocomial pneumonia: a window of opportunity for pharmacokinetic and pharmacodynamic modeling.Expert Opin Drug Metab Toxicol. 2023 Jan;19(1):13-25. doi: 10.1080/17425255.2023.2178896. Epub 2023 Feb 18. Expert Opin Drug Metab Toxicol. 2023. PMID: 36786064 Review.
-
Polymyxin combination therapy for multidrug-resistant, extensively-drug resistant, and difficult-to-treat drug-resistant gram-negative infections: is it superior to polymyxin monotherapy?Expert Rev Anti Infect Ther. 2023 Apr;21(4):387-429. doi: 10.1080/14787210.2023.2184346. Epub 2023 Mar 8. Expert Rev Anti Infect Ther. 2023. PMID: 36820511 Review.
Cited by
-
The efficacy of polymyxin B in treating stroke-associated pneumonia with carbapenem-resistant Gram-negative bacteria infections: a multicenter real-world study using propensity score matching.Front Pharmacol. 2025 Mar 20;16:1413563. doi: 10.3389/fphar.2025.1413563. eCollection 2025. Front Pharmacol. 2025. PMID: 40183094 Free PMC article.
-
Efficacy and Safety Factors Related to Plasma Concentration-Optimized Polymyxin B Therapy in Treating Carbapenem-Resistant Gram-Negative Bacterial Infections in China.Infect Drug Resist. 2024 Jul 16;17:3057-3071. doi: 10.2147/IDR.S468890. eCollection 2024. Infect Drug Resist. 2024. PMID: 39050834 Free PMC article.
-
Risk Factors Associated with Antibiotic Exposure Variability in Critically Ill Patients: A Systematic Review.Antibiotics (Basel). 2024 Aug 24;13(9):801. doi: 10.3390/antibiotics13090801. Antibiotics (Basel). 2024. PMID: 39334976 Free PMC article. Review.
-
Efficiency of polymyxin B treatment against nosocomial infection: a systematic review and meta-analysis.Front Med (Lausanne). 2024 May 28;11:1400757. doi: 10.3389/fmed.2024.1400757. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38863886 Free PMC article.
-
The challenges of difficult-to-treat Acinetobacter infections.Clin Microbiol Rev. 2024 Dec 10;37(4):e0009324. doi: 10.1128/cmr.00093-24. Epub 2024 Nov 18. Clin Microbiol Rev. 2024. PMID: 39555919 Review.
References
-
- Paul M, Carrara E, Retamar P, Tangden T, Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S, et al. European society of clinical microbiology and infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine) Clin Microbiol Infect. 2022;28(4):521–547. doi: 10.1016/j.cmi.2021.11.025. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

