Circulating Extracellular-Vesicle-Incorporated MicroRNAs as Potential Biomarkers for Ischemic Stroke in Patients With Cancer
- PMID: 37106564
- PMCID: PMC10250879
- DOI: 10.5853/jos.2022.02327
Circulating Extracellular-Vesicle-Incorporated MicroRNAs as Potential Biomarkers for Ischemic Stroke in Patients With Cancer
Abstract
Background and purpose: This study aimed to evaluate whether extracellular-vesicle-incorporated microRNAs (miRNAs) are potential biomarkers for cancer-related stroke.
Methods: This cohort study compared patients with active cancer who had embolic stroke of unknown sources (cancer-stroke group) with patients with only cancer, patients with only stroke, and healthy individuals (control groups). The expression profiles of miRNAs encapsulated in plasma exosomes and microvesicles were evaluated using microarray and validated using quantitative real-time polymerase chain reaction. The XENO-QTM miRNA assay technology was used to determine the absolute copy numbers of individual miRNAs in an external validation cohort.
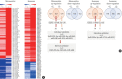
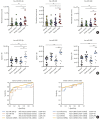
Results: This study recruited 220 patients, of which 45 had cancer-stroke, 76 were healthy controls, 39 were cancer controls, and 60 were stroke controls. Three miRNAs (miR-205-5p, miR-645, and miR-646) were specifically incorporated into microvesicles in patients with cancer-related stroke, cancer controls, and stroke controls. The area under the receiver operating characteristic curves of these three miRNAs were 0.7692-0.8510 for the differentiation of patients with cancer-stroke from cancer-controls and 0.8077-0.8846 for the differentiation of patients with cancer-stroke from stroke controls. The levels of several miRNAs were elevated in the plasma exosomes of patients with cancer, but were lower than those in plasma microvesicles. An in vivo study showed that systemic injection of miR-205-5p promoted the development of arterial thrombosis and elevation of D-dimer levels.
Conclusion: Stroke due to cancer-related coagulopathy was associated with deregulated expression of miRNAs, particularly microvesicle-incorporated miR-205-5p, miR-645, and miR-646. Further prospective studies of extracellular-vesicle-incorporated miRNAs are required to confirm the diagnostic role of miRNAs in patients with stroke and to screen the roles of miRNAs in patients with cancer.
Keywords: Biomarker; Cancer; Coagulopathy; Extracellular vesicle; MicroRNA; Stroke.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures






Similar articles
-
Serum outperforms plasma in small extracellular vesicle microRNA biomarker studies of adenocarcinoma of the esophagus.World J Gastroenterol. 2020 May 28;26(20):2570-2583. doi: 10.3748/wjg.v26.i20.2570. World J Gastroenterol. 2020. PMID: 32523312 Free PMC article.
-
MicroRNA Expression Profile in Acute Ischemic Stroke.Res Sq [Preprint]. 2024 Jan 3:rs.3.rs-3754883. doi: 10.21203/rs.3.rs-3754883/v1. Res Sq. 2024. Update in: Int J Mol Sci. 2025 Jan 17;26(2):747. doi: 10.3390/ijms26020747. PMID: 38260305 Free PMC article. Updated. Preprint.
-
Circulating microRNAs from the miR-106a-363 cluster on chromosome X as novel diagnostic biomarkers for breast cancer.Breast Cancer Res Treat. 2018 Jul;170(2):257-270. doi: 10.1007/s10549-018-4757-3. Epub 2018 Mar 20. Breast Cancer Res Treat. 2018. PMID: 29557526 Free PMC article.
-
A panel of seven-miRNA signature in plasma as potential biomarker for colorectal cancer diagnosis.Gene. 2019 Mar 1;687:246-254. doi: 10.1016/j.gene.2018.11.055. Epub 2018 Nov 17. Gene. 2019. PMID: 30458288
-
Atherosclerosis-related circulating miRNAs as novel and sensitive predictors for acute myocardial infarction.PLoS One. 2014 Sep 3;9(9):e105734. doi: 10.1371/journal.pone.0105734. eCollection 2014. PLoS One. 2014. PMID: 25184815 Free PMC article.
Cited by
-
Machine Learning-Based Etiologic Subtyping of Ischemic Stroke Using Circulating Exosomal microRNAs.Int J Mol Sci. 2024 Jun 20;25(12):6761. doi: 10.3390/ijms25126761. Int J Mol Sci. 2024. PMID: 38928481 Free PMC article.
-
Expression profile of circulating miRNAs in patients with atrial fibrillation-dominated cardioembolic stroke: A systematic review and bioinformatics analysis.Heliyon. 2024 Jul 25;10(15):e35201. doi: 10.1016/j.heliyon.2024.e35201. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39166047 Free PMC article.
-
Knowledge mapping of exosomes in ischemic stroke: a bibliometric analysis.Front Neurol. 2025 Jul 23;16:1595379. doi: 10.3389/fneur.2025.1595379. eCollection 2025. Front Neurol. 2025. PMID: 40771978 Free PMC article.
-
A case-control comparison of acute-phase peripheral blood gene expression in participants diagnosed with minor ischaemic stroke or stroke mimics.Hum Genomics. 2023 Nov 25;17(1):106. doi: 10.1186/s40246-023-00551-y. Hum Genomics. 2023. PMID: 38007520 Free PMC article.
-
Simultaneous and rapid colorimetric detection of distinct miRNAs using Split-LAMP.Front Bioeng Biotechnol. 2023 Nov 2;11:1271297. doi: 10.3389/fbioe.2023.1271297. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38026864 Free PMC article.
References
-
- Ohara T, Farhoudi M, Bang OY, Koga M, Demchuk AM. The emerging value of serum D-dimer measurement in the workup and management of ischemic stroke. Int J Stroke. 2020;15:122–131. - PubMed
-
- Nam GH, Choi Y, Kim GB, Kim S, Kim SA, Kim IS. Emerging prospects of exosomes for cancer treatment: from conventional therapy to immunotherapy. Adv Mater. 2020;32:e2002440. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

