Improving Bioactive Characteristics of Small Diameter Polytetrafluoroethylene Stent Grafts by Electrospinning: A Comparative Hemocompatibility Study
- PMID: 37106598
- PMCID: PMC10135465
- DOI: 10.3390/bioengineering10040411
Improving Bioactive Characteristics of Small Diameter Polytetrafluoroethylene Stent Grafts by Electrospinning: A Comparative Hemocompatibility Study
Abstract
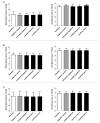
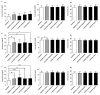
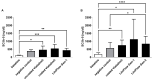
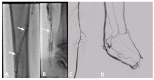
Polytetrafluoroethylene (PTFE) is a commonly used biomaterial for the manufacturing of vascular grafts and several strategies, such as coatings, have been explored to improve the hemocompatibility of small-diameter prostheses. In this study, the hemocompatibility properties of novel stent grafts covered with electrospun PTFE (LimFlow Gen-1 and LimFlow Gen-2) were compared with uncoated and heparin-coated PTFE grafts (Gore Viabahn®) using fresh human blood in a Chandler closed-loop system. After 60 min of incubation, the blood samples were examined hematologically and activation of coagulation, platelets, and the complement system were analyzed. In addition, the adsorbed fibrinogen on the stent grafts was measured and the thrombogenicity was assessed by SEM. Significantly lower adsorption of fibrinogen was measured on the surface of heparin-coated Viabahn than on the surface of the uncoated Viabahn. Furthermore, LimFlow Gen-1 stent grafts showed lower fibrinogen adsorption than the uncoated Viabahn®, and the LimFlow Gen-2 stent grafts showed comparable fibrinogen adsorption as the heparin-coated Viabahn®. SEM analysis revealed no sign of thrombus formation on any of the stent surfaces. LimFlow Gen-2 stent grafts covered with electrospun PTFE exhibited bioactive characteristics and revealed improved hemocompatibility in terms of reduced adhesion of fibrinogen, activation of platelets, and coagulation (assessed by β-TG and TAT levels) similar to heparin-coated ePTFE prostheses. Thus, this study demonstrated improved hemocompatibility of electrospun PTFE. The next step is to conduct in vivo studies to confirm whether electrospinning-induced changes to the PTFE surface can reduce the risk of thrombus formation and provide clinical benefits.
Keywords: electrospinning; hemocompatibility; polytetrafluoroethylene stent graft.
Conflict of interest statement
The authors disclosure that this study was sponsored by company. V.C. is working as a consultant for LimFlow SA.
Figures







References
-
- Canjuga D., Hansen C., Halbrügge F., Hann L., Weiß S., Schlensak C., Wendel H.-P., Avci-Adali M. Improving hemocompatibility of artificial lungs by click conjugation of glycoengineered endothelial cells onto blood-contacting surfaces. Biomater. Adv. 2022;137:212824. doi: 10.1016/j.bioadv.2022.212824. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

