Inhibitors of ATP Synthase as New Antibacterial Candidates
- PMID: 37107012
- PMCID: PMC10135114
- DOI: 10.3390/antibiotics12040650
Inhibitors of ATP Synthase as New Antibacterial Candidates
Abstract
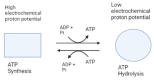
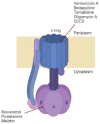
ATP, the power of all cellular functions, is constantly used and produced by cells. The enzyme called ATP synthase is the energy factory in all cells, which produces ATP by adding inorganic phosphate (Pi) to ADP. It is found in the inner, thylakoid and plasma membranes of mitochondria, chloroplasts and bacteria, respectively. Bacterial ATP synthases have been the subject of multiple studies for decades, since they can be genetically manipulated. With the emergence of antibiotic resistance, many combinations of antibiotics with other compounds that enhance the effect of these antibiotics have been proposed as approaches to limit the spread of antibiotic-resistant bacteria. ATP synthase inhibitors, such as resveratrol, venturicidin A, bedaquiline, tomatidine, piceatannol, oligomycin A and N,N-dicyclohexylcarbodiimide were the starting point of these combinations. However, each of these inhibitors target ATP synthase differently, and their co-administration with antibiotics increases the susceptibility of pathogenic bacteria. After a brief description of the structure and function of ATP synthase, we aim in this review to highlight therapeutic applications of the major bacterial ATP synthase inhibitors, including animal's venoms, and to emphasize their importance in decreasing the activity of this enzyme and subsequently eradicating resistant bacteria as ATP synthase is their source of energy.
Keywords: ATP synthase; ATP synthase inhibitors; animal venoms; resistant bacteria; therapeutic application.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

