Physiologically-Based Pharmacokinetic Modelling to Predict the Pharmacokinetics and Pharmacodynamics of Linezolid in Adults and Children with Tuberculous Meningitis
- PMID: 37107064
- PMCID: PMC10135070
- DOI: 10.3390/antibiotics12040702
Physiologically-Based Pharmacokinetic Modelling to Predict the Pharmacokinetics and Pharmacodynamics of Linezolid in Adults and Children with Tuberculous Meningitis
Abstract
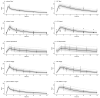
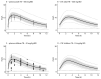
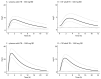
Linezolid is used off-label for treatment of central nervous system infections. However, its pharmacokinetics and target attainment in cranial cerebrospinal fluid (CSF) in tuberculous meningitis patients is unknown. This study aimed to predict linezolid cranial CSF concentrations and assess attainment of pharmacodynamic (PD) thresholds (AUC:MIC of >119) in plasma and cranial CSF of adults and children with tuberculous meningitis. A physiologically based pharmacokinetic (PBPK) model was developed to predict linezolid cranial CSF profiles based on reported plasma concentrations. Simulated steady-state PK curves in plasma and cranial CSF after linezolid doses of 300 mg BID, 600 mg BID, and 1200 mg QD in adults resulted in geometric mean AUC:MIC ratios in plasma of 118, 281, and 262 and mean cranial CSF AUC:MIC ratios of 74, 181, and 166, respectively. In children using ~10 mg/kg BID linezolid, AUC:MIC values at steady-state in plasma and cranial CSF were 202 and 135, respectively. Our model predicts that 1200 mg per day in adults, either 600 mg BID or 1200 mg QD, results in reasonable (87%) target attainment in cranial CSF. Target attainment in our simulated paediatric population was moderate (56% in cranial CSF). Our PBPK model can support linezolid dose optimization efforts by simulating target attainment close to the site of TBM disease.
Keywords: PBPK; linezolid; tuberculous meningitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pfizer Inc. (per FDA) Product Information: ZYVOX(R) Intravenous Injection, Oral Tablets Suspension, Linezolid Intravenous Injection, Oral Tablets Suspension; New York, NY, USA. [(accessed on 29 March 2023)];2013 Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021130s032,021....
-
- WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment. 2019. [(accessed on 29 March 2023)]. Available online: https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-re...
-
- Beer R., Engelhardt K.W., Pfausler B., Broessner G., Helbok R., Lackner P., Brenneis C., Kaehler S.T., Georgopoulos A., Schmutzhard E. Pharmacokinetics of Intravenous Linezolid in Cerebrospinal Fluid and Plasma in Neurointensive Care Patients with Staphylococcal Ventriculitis Associated with External Ventricular Drains. Antimicrob. Agents Chemother. 2007;51:379–382. doi: 10.1128/AAC.00515-06. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

