Development, Optimization, and In Vitro/In Vivo Evaluation of Azelaic Acid Transethosomal Gel for Antidermatophyte Activity
- PMID: 37107069
- PMCID: PMC10135108
- DOI: 10.3390/antibiotics12040707
Development, Optimization, and In Vitro/In Vivo Evaluation of Azelaic Acid Transethosomal Gel for Antidermatophyte Activity
Abstract
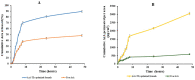
Treatment of dermatophytosis is quite challenging. This work aims to investigate the antidermatophyte action of Azelaic acid (AzA) and evaluate its efficacy upon entrapment into transethosomes (TEs) and incorporation into a gel to enhance its application. Optimization of formulation variables of TEs was carried out after preparation using the thin film hydration technique. The antidermatophyte activity of AzA-TEs was first evaluated in vitro. In addition, two guinea pig infection models with Trichophyton (T.) mentagrophytes and Microsporum (M.) canis were established for the in vivo assessment. The optimized formula showed a mean particle size of 219.8 ± 4.7 nm and a zeta potential of -36.5 ± 0.73 mV, while the entrapment efficiency value was 81.9 ± 1.4%. Moreover, the ex vivo permeation study showed enhanced skin penetration for the AzA-TEs (3056 µg/cm2) compared to the free AzA (590 µg/cm2) after 48 h. AzA-TEs induced a greater inhibition in vitro on the tested dermatophyte species than free AzA (MIC90 was 0.01% vs. 0.32% for T. rubrum and 0.032% for T. mentagrophytes and M. canis vs. 0.56%). The mycological cure rate was improved in all treated groups, specially for our optimized AzA-TEs formula in the T. mentagrophytes model, in which it reached 83% in this treated group, while it was 66.76% in the itraconazole and free AzA treated groups. Significant (p < 0.05) lower scores of erythema, scales, and alopecia were observed in the treated groups in comparison with the untreated control and plain groups. In essence, the TEs could be a promising carrier for AzA delivery into deeper skin layers with enhanced antidermatophyte activity.
Keywords: Microsporum canis; Trichophyton mentagrophytes; antidermatophyte; azelaic acid; guinea pig model; thin film hydration; transethosomes.
Conflict of interest statement
The authors declare no conflict of interest.
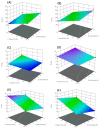
Figures



References
LinkOut - more resources
Full Text Sources

