Mitochondrial ROS Triggers KIN Pathogenesis in FAN1-Deficient Kidneys
- PMID: 37107275
- PMCID: PMC10135478
- DOI: 10.3390/antiox12040900
Mitochondrial ROS Triggers KIN Pathogenesis in FAN1-Deficient Kidneys
Abstract
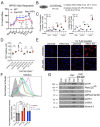
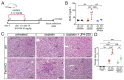
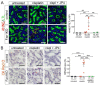
Karyomegalic interstitial nephritis (KIN) is a genetic adult-onset chronic kidney disease (CKD) characterized by genomic instability and mitotic abnormalities in the tubular epithelial cells. KIN is caused by recessive mutations in the FAN1 DNA repair enzyme. However, the endogenous source of DNA damage in FAN1/KIN kidneys has not been identified. Here we show, using FAN1-deficient human renal tubular epithelial cells (hRTECs) and FAN1-null mice as a model of KIN, that FAN1 kidney pathophysiology is triggered by hypersensitivity to endogenous reactive oxygen species (ROS), which cause chronic oxidative and double-strand DNA damage in the kidney tubular epithelial cells, accompanied by an intrinsic failure to repair DNA damage. Furthermore, persistent oxidative stress in FAN1-deficient RTECs and FAN1 kidneys caused mitochondrial deficiencies in oxidative phosphorylation and fatty acid oxidation. The administration of subclinical, low-dose cisplatin increased oxidative stress and aggravated mitochondrial dysfunction in FAN1-deficient kidneys, thereby exacerbating KIN pathophysiology. In contrast, treatment of FAN1 mice with a mitochondria-targeted ROS scavenger, JP4-039, attenuated oxidative stress and accumulation of DNA damage, mitigated tubular injury, and preserved kidney function in cisplatin-treated FAN1-null mice, demonstrating that endogenous oxygen stress is an important source of DNA damage in FAN1-deficient kidneys and a driver of KIN pathogenesis. Our findings indicate that therapeutic modulation of kidney oxidative stress may be a promising avenue to mitigate FAN1/KIN kidney pathophysiology and disease progression in patients.
Keywords: DNA damage; FAN1; chronic kidney disease; karyomegalic interstitial nephritis; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Zhou W., Otto E.A., Cluckey A., Airik R., Hurd T.W., Chaki M., Diaz K., Lach F.P., Bennett G.R., Gee H.Y., et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat. Genet. 2012;44:910–915. doi: 10.1038/ng.2347. - DOI - PMC - PubMed
-
- Spoendlin M., Moch H., Brunner F., Brunner W., Burger H.R., Kiss D., Wegmann W., Dalquen P., Oberholzer M., Thiel G., et al. Karyomegalic interstitial nephritis: Further support for a distinct entity and evidence for a genetic defect. Am. J. Kidney Dis. 1995;25:242–252. doi: 10.1016/0272-6386(95)90005-5. - DOI - PubMed
-
- Gueguen L., Delaval R., Blanluet M., Sartelet H., Leou S., Dubois d’Enghien C., Golmard L., Stoppa-Lyonnet D., Testevuide P., Faguer S. Recurrent FAN1 p.W707X Pathogenic Variant Originating Before ad 1800 Underlies High Frequency of Karyomegalic Interstitial Nephritis in South Pacific Islands. Kidney Int. Rep. 2021;6:2207–2211. doi: 10.1016/j.ekir.2021.05.010. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

