Agomelatine, a Melatonin-Derived Drug, as a New Strategy for the Treatment of Colorectal Cancer
- PMID: 37107301
- PMCID: PMC10135458
- DOI: 10.3390/antiox12040926
Agomelatine, a Melatonin-Derived Drug, as a New Strategy for the Treatment of Colorectal Cancer
Abstract
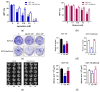
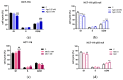
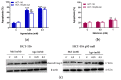
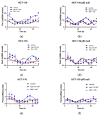
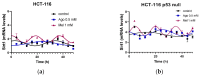
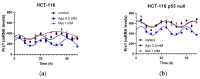
The potential use of agomelatine as an alternative treatment for colorectal cancer is evaluated in this work. The effect of agomelatine was studied in an in vitro model using two cell lines with different p53 statuses (HCT-116, wild-type p53, and HCT-116 p53 null) and an in vivo xenograft model. The inhibitory effects of agomelatine and melatonin were stronger in the cells harboring the wild-type p53, although in both cell lines, the effect of agomelatine was greater than that of the melatonin. In vivo, only agomelatine was able to reduce the volumes of tumors generated by the HCT-116-p53-null cells. Both treatments induced changes in the rhythmicity of the circadian-clock genes in vitro, albeit with some differences. Agomelatine and melatonin regulated the rhythmicity of Per1-3, Cry1, Sirt1, and Prx1 in the HCT-116 cells. In these cells, agomelatine also regulated Bmal1 and Nr1d2, while melatonin changed the rhythmicity of Clock. In the HCT-116-p53-null cells, agomelatine regulated Per1-3, Cry1, Clock, Nr1d2, Sirt1, and Prx1; however, melatonin only induced changes in Clock, Bmal1, and Sirt1. The differences found in the regulation of the clock genes may explain the greater oncostatic effect of agomelatine in CRC.
Keywords: SIRT1; agomelatine; circadian clock; colorectal cancer; melatonin; p53.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

