Effects of Ficus pandurata Hance var. angustifolia Cheng Flavonoids on Intestinal Barrier and Cognitive Function by Regulating Intestinal Microbiota
- PMID: 37107477
- PMCID: PMC10137925
- DOI: 10.3390/foods12081682
Effects of Ficus pandurata Hance var. angustifolia Cheng Flavonoids on Intestinal Barrier and Cognitive Function by Regulating Intestinal Microbiota
Abstract
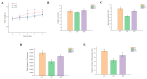
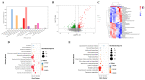
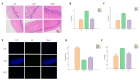
More and more evidence has supported the interaction between circadian rhythms and intestinal microbes, which provides new insights into how dietary nutrition can improve host health. Our research showed that Ficus pandurata Hance var. angustifolia Cheng flavonoids (FCF) ameliorated the pathological damage of colon and abnormal intestinal microflora structure in mice with circadian clock disorder and improved their exploration and memory behaviors. Mechanism studies have shown that FCF is involved in regulating metabolic pathways and related metabolites, regulating the expression of related tight junction proteins in the colon and the levels of Aβ and inflammatory factors in the hippocampus. Further analysis found that these metabolites showed a certain correlation with intestinal flora and played a certain role in alleviating intestinal physiological damage and cognitive decline.
Keywords: Ficus pandurata Hance var. angustifolia Cheng flavonoids; cognitive function; intestinal barrier; intestinal microbiota.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Leone V., Gibbons S.M., Martinez K., Hutchison A.L., Huang E.Y., Cham C.M., Pierre J.F., Heneghan A.F., Nadimpalli A., Hubert N., et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe. 2015;17:681–689. doi: 10.1016/j.chom.2015.03.006. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

