Damage-Free Shortening of Telomeres Is a Potential Strategy Supporting Blind Mole-Rat Longevity
- PMID: 37107603
- PMCID: PMC10137574
- DOI: 10.3390/genes14040845
Damage-Free Shortening of Telomeres Is a Potential Strategy Supporting Blind Mole-Rat Longevity
Abstract
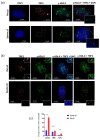
Telomere shortening or loss of shelterin components activates DNA damage response (DDR) pathways, leading to a replicative senescence that is usually coupled with a senescence-associated secretory phenotype (SASP). Recent studies suggested that telomere aberration that activates DDR may occur, irrespective of telomere length or loss of shelterin complex. The blind mole-rat (Spalax) is a subterranean rodent with exceptional longevity, and its cells demonstrate an uncoupling of senescence and SASP inflammatory components. Herein, we evaluated Spalax relative telomere length, telomerase activity, and shelterin expression, along with telomere-associated DNA damage foci (TAFs) levels with cell passage. We show that telomeres shorten in Spalax fibroblasts similar to the process in rats, and that the telomerase activity is lower. Moreover, we found lower DNA damage foci at the telomeres and a decline in the mRNA expression of two shelterin proteins, known as ATM/ATR repressors. Although additional studies are required for understanding the underling mechanism, our present results imply that Spalax genome protection strategies include effective telomere maintenance, preventing early cellular senescence induced by persistent DDR, thereby contributing to its longevity and healthy aging.
Keywords: DNA damage; Spalax; senescence; shelterin; telomerase activity; telomere length.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

