Leri-Weill Dyschondrosteosis Caused by a Leaky Homozygous SHOX Splice-Site Variant
- PMID: 37107635
- PMCID: PMC10138022
- DOI: 10.3390/genes14040877
Leri-Weill Dyschondrosteosis Caused by a Leaky Homozygous SHOX Splice-Site Variant
Abstract
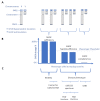
SHOX deficiency is a common genetic cause of short stature of variable degree. SHOX haploinsufficiency causes Leri-Weill dyschondrosteosis (LWD) as well as nonspecific short stature. SHOX haploinsufficiency is known to result from heterozygous loss-of-function variants with pseudo-autosomal dominant inheritance, while biallelic SHOX loss-of-function variants cause the more severe skeletal dysplasia, Langer mesomelic dyschondrosteosis (LMD). Here we report for the first time the pseudo-autosomal recessive inheritance of LWD in two siblings caused by a novel homozygous non-canonical, leaky splice-site variant in intron 3 of SHOX: c.544+5G>C. Transcript analyses in patient-derived fibroblasts showed homozygous patients to produce approximately equal amounts of normally spliced mRNA and mRNA with the abnormal retention of intron 3 and containing a premature stop codon (p.Val183Glyfs*31). The aberrant transcript was shown to undergo nonsense-mediated mRNA decay, and thus resulting in SHOX haploinsufficiency in the homozygous patient. Six healthy relatives who are of normal height are heterozygous for this variant and fibroblasts from a heterozygote for the c.544+5G>C variant produced wild-type transcript amounts comparable to healthy control. The unique situation reported here highlights the fact that the dosage of SHOX determines the clinical phenotype rather than the Mendelian inheritance pattern of SHOX variants. This study extends the molecular and inheritance spectrum of SHOX deficiency disorder and highlights the importance of functional testing of SHOX variants of unknown significance in order to allow appropriate counseling and precision medicine for each family individual.
Keywords: SHOX; gene dosage; haploinsufficiency; leaky splice-site; precision medicine; pseudo-autosomal inheritance; short stature; skeletal disorder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rao E., Weiss B., Fukami M., Rump A., Niesler B., Mertz A., Muroya K., Binder G., Kirsch S., Winkelmann M., et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat. Genet. 1997;16:54–63. doi: 10.1038/ng0597-54. - DOI - PubMed
-
- Marchini A., Marttila T., Winter A., Caldeira S., Malanchi I., Blaschke R.J., Häcker B., Rao E., Karperien M., Wit J.M., et al. The short stature homeodomain protein SHOX induces cellular growth arrest and apoptosis and is expressed in human growth plate chondrocytes. J. Biol. Chem. 2004;279:37103–37114. doi: 10.1074/jbc.M307006200. - DOI - PubMed
-
- Binder G., Rappold G.A. SHOX Deficiency Disorders. In: Adam M.P., Everman D.B., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. GeneReviews((R)) University of Washington; Seattle, WA, USA: 1993. - PubMed
-
- Ottesen A.M., Aksglaede L., Garn I., Tartaglia N., Tassone F., Gravholt C.H., Bojesen A., Sorensen K., Jorgensen N., Rajpert-De Meyts E., et al. Increased number of sex chromosomes affects height in a nonlinear fashion: A study of 305 patients with sex chromosome aneuploidy. Am. J. Med. Genet. A. 2010;152A:1206–1212. doi: 10.1002/ajmg.a.33334. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

