Differential Impact of Intermittent vs. Sustained Hypoxia on HIF-1, VEGF and Proliferation of HepG2 Cells
- PMID: 37108039
- PMCID: PMC10139223
- DOI: 10.3390/ijms24086875
Differential Impact of Intermittent vs. Sustained Hypoxia on HIF-1, VEGF and Proliferation of HepG2 Cells
Abstract
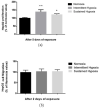
Obstructive sleep apnea (OSA) is an emerging risk factor for cancer occurrence and progression, mainly mediated by intermittent hypoxia (IH). Systemic IH, a main landmark of OSA, and local sustained hypoxia (SH), a classical feature at the core of tumors, may act separately or synergistically on tumor cells. Our aim was to compare the respective consequences of intermittent and sustained hypoxia on HIF-1, endothelin-1 and VEGF expression and on cell proliferation and migration in HepG2 liver tumor cells. Wound healing, spheroid expansion, proliferation and migration were evaluated in HepG2 cells following IH or SH exposure. The HIF-1α, endothelin-1 and VEGF protein levels and/or mRNA expression were assessed, as were the effects of HIF-1 (acriflavine), endothelin-1 (macitentan) and VEGF (pazopanib) inhibition. Both SH and IH stimulated wound healing, spheroid expansion and proliferation of HepG2 cells. HIF-1 and VEGF, but not endothelin-1, expression increased with IH exposure but not with SH exposure. Acriflavine prevented the effects of both IH and SH, and pazopanib blocked those of IH but not those of SH. Macitentan had no impact. Thus, IH and SH stimulate hepatic cancer cell proliferation via distinct signaling pathways that may act synergistically in OSA patients with cancer, leading to enhanced tumor progression.
Keywords: HepG2; hypoxia inducible factor 1 (HIF-1); intermittent hypoxia (IH); liver cancer; obstructive sleep apnea syndrome (OSA); sustained hypoxia (SH); vascular endothelial growth factor (VEGF).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Benjafield A.V., Ayas N.T., Eastwood P.R., Heinzer R., Ip M.S.M., Morrell M.J., Nunez C.M., Patel S.R., Penzel T., Pépin J.-L., et al. Estimation of the Global Prevalence and Burden of Obstructive Sleep Apnoea: A Literature-Based Analysis. Lancet Respir. Med. 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. - DOI - PMC - PubMed
-
- Justeau G., Gervès-Pinquié C., le Vaillant M., Trzepizur W., Meslier N., Goupil F., Pigeanne T., Launois S., Le-clair-Visonneau L., Masson P., et al. Association between Nocturnal Hypoxemia and Cancer Incidence in Patients Investigated for OSA: Data from a Large Multicenter French Cohort. Chest. 2020;158:2610–2620. doi: 10.1016/j.chest.2020.06.055. - DOI - PubMed
-
- Campos-Rodriguez F., Martinez-Garcia M.A., Martinez M., Duran-Cantolla J., de La Penã M., Masdeu M.J., Gon-zalez M., del Campo F., Gallego I., Marin J.M., et al. Association between Obstructive Sleep Apnea and Cancer Incidence in a Large Multicenter Spanish Cohort. Am. J. Respir. Crit. Care Med. 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

