How Can We Prevent Mother-to-Child Transmission of HTLV-1?
- PMID: 37108125
- PMCID: PMC10138424
- DOI: 10.3390/ijms24086961
How Can We Prevent Mother-to-Child Transmission of HTLV-1?
Abstract
The perception of human T-cell leukemia virus type 1 (HTlV-1) infection as a "silent disease" has recently given way to concern that its presence may be having a variety of effects. HTLV-1 is known to cause adult T-cell leukemia (ATL), an aggressive cancer of peripheral CD4 T cells; however, it is also responsible for HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Most patients develop ATL as a result of HTLV-1 mother-to-child transmission. The primary route of mother-to-child transmission is through the mother's milk. In the absence of effective drug therapy, total artificial nutrition such as exclusive formula feeding is a reliable means of preventing mother-to-child transmission after birth, except for a small percentage of prenatal infections. A recent study found that the rate of mother-to-child transmission with short-term breastfeeding (within 90 days) did not exceed that of total artificial nutrition. Because these preventive measures are in exchange for the benefits of breastfeeding, clinical applications of antiretroviral drugs and immunotherapy with vaccines and neutralizing antibodies are urgently needed.
Keywords: adult T-cell leukemia (ATL); antenatal screening; human T-cell leukemia virus type 1 (HTlV-1); mother-to-child transmission; nutritional regimens; prevention.
Conflict of interest statement
The authors declare no conflict of interest associated with this manuscript.
Figures



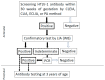
Similar articles
-
Cost-effectiveness of human T-cell leukemia virus type 1 (HTLV-1) antenatal screening for prevention of mother-to-child transmission.PLoS Negl Trop Dis. 2023 Feb 21;17(2):e0011129. doi: 10.1371/journal.pntd.0011129. eCollection 2023 Feb. PLoS Negl Trop Dis. 2023. PMID: 36809372 Free PMC article.
-
Early Onset of HTLV-1 Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) and Adult T-cell Leukemia/Lymphoma (ATL): Systematic Search and Review.J Trop Pediatr. 2018 Apr 1;64(2):151-161. doi: 10.1093/tropej/fmx039. J Trop Pediatr. 2018. PMID: 28582585
-
Could Cesarean Delivery Help Prevent Mother-to-Child Transmission of Human T-Lymphotropic Virus Type 1?J Infect Dis. 2023 Dec 20;228(12):1766-1775. doi: 10.1093/infdis/jiad219. J Infect Dis. 2023. PMID: 37386934 Review.
-
HTLV-I serostatus of mothers of patients with adult T-cell leukemia and HTLV-I-associated myelopathy/tropical spastic paraparesis.J Hum Virol. 1998 May-Jun;1(4):302-5. J Hum Virol. 1998. PMID: 10195256
-
Adult T-cell leukemia/lymphoma and HTLV-1.Curr Hematol Malig Rep. 2007 Oct;2(4):257-64. doi: 10.1007/s11899-007-0035-x. Curr Hematol Malig Rep. 2007. PMID: 20425378 Review.
Cited by
-
Human T-Lymphotropic Virus (HTLV): Epidemiology, Genetic, Pathogenesis, and Future Challenges.Viruses. 2025 May 1;17(5):664. doi: 10.3390/v17050664. Viruses. 2025. PMID: 40431676 Free PMC article. Review.
-
HTLV-1 in Pregnancy and Neonatal Health: Evidence, Challenges, and Future Directions.Diagnostics (Basel). 2025 Jul 28;15(15):1886. doi: 10.3390/diagnostics15151886. Diagnostics (Basel). 2025. PMID: 40804851 Free PMC article. Review.
-
Distinct characteristics and social determinants in adult T-cell leukaemia/lymphoma patients at a tertiary cancer centre in Canada.Br J Haematol. 2025 Jul;207(1):132-140. doi: 10.1111/bjh.20132. Epub 2025 May 6. Br J Haematol. 2025. PMID: 40328512 Free PMC article.
-
The Interactions between Cells and Viruses.Int J Mol Sci. 2024 Jun 23;25(13):6886. doi: 10.3390/ijms25136886. Int J Mol Sci. 2024. PMID: 38999995 Free PMC article.
-
Silent dissemination of HTLV-1: evidence of intrafamilial transmission in a Brazilian reference centre.Mem Inst Oswaldo Cruz. 2025 Mar 31;120:e240191. doi: 10.1590/0074-02760240191. eCollection 2025. Mem Inst Oswaldo Cruz. 2025. PMID: 40172427 Free PMC article.
References
-
- Gallo R.C. Kyoto Workshop on some specific recent advances in human tumor virology. Cancer Res. 1981;41:4738–4739. - PubMed
-
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K., Shirakawa S., Miyoshi I. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

