ZnO Nanorods Create a Hypoxic State with Induction of HIF-1 and EPAS1, Autophagy, and Mitophagy in Cancer and Non-Cancer Cells
- PMID: 37108134
- PMCID: PMC10138614
- DOI: 10.3390/ijms24086971
ZnO Nanorods Create a Hypoxic State with Induction of HIF-1 and EPAS1, Autophagy, and Mitophagy in Cancer and Non-Cancer Cells
Abstract
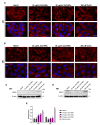
Nanomaterials are gaining increasing attention as innovative materials in medicine. Among nanomaterials, zinc oxide (ZnO) nanostructures are particularly appealing because of their opto-electrical, antimicrobial, and photochemical properties. Although ZnO is recognized as a safe material and the Zn ion (Zn2+) concentration is strictly regulated at a cellular and systemic level, different studies have demonstrated cellular toxicity of ZnO nanoparticles (ZnO-NPs) and ZnO nanorods (ZnO-NRs). Recently, ZnO-NP toxicity has been shown to depend on the intracellular accumulation of ROS, activation of autophagy and mitophagy, as well as stabilization and accumulation of hypoxia-inducible factor-1α (HIF-1α) protein. However, if the same pathway is also activated by ZnO-NRs and how non-cancer cells respond to ZnO-NR treatment, are still unknown. To answer to these questions, we treated epithelial HaCaT and breast cancer MCF-7 cells with different ZnO-NR concentrations. Our results showed that ZnO-NR treatments increased cell death through ROS accumulation, HIF-1α and endothelial PAS domain protein 1 (EPAS1) activation, and induction of autophagy and mitophagy in both cell lines. These results, while on one side, confirmed that ZnO-NRs can be used to reduce cancer growth, on the other side, raised some concerns on the activation of a hypoxic response in normal cells that, in the long run, could induce cellular transformation.
Keywords: ZnO nanorods; autophagy; cancer; hypoxia; mitophagy; nanomaterials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Ravi S., Bajpai V. Recent advances in ZnO nanostructures and their future perspective. Adv. Nano Res. 2021;11:37–54. doi: 10.12989/anr.2021.11.1.037. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

