Tumor Necrosis Factor Superfamily 14 (LIGHT) Restricts Neovascularization by Decreasing Circulating Endothelial Progenitor Cells and Function
- PMID: 37108160
- PMCID: PMC10138919
- DOI: 10.3390/ijms24086997
Tumor Necrosis Factor Superfamily 14 (LIGHT) Restricts Neovascularization by Decreasing Circulating Endothelial Progenitor Cells and Function
Abstract
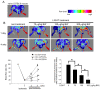
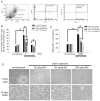
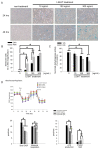
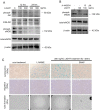
Tumor necrosis factor superfamily 14 (TNFSF14) is also known as the LT-related inducible ligand (LIGHT). It can bind to the herpesvirus invasion mediator and lymphotoxin-β receptor to perform its biological activity. LIGHT has multiple physiological functions, including strengthening the synthesis of nitric oxide, reactive oxygen species, and cytokines. LIGHT also stimulates angiogenesis in tumors and induces the synthesis of high endothelial venules; degrades the extracellular matrix in thoracic aortic dissection, and induces the expression of interleukin-8, cyclooxygenase-2, and cell adhesion molecules in endothelial cells. While LIGHT induces tissue inflammation, its effects on angiogenesis after tissue ischemia are unclear. Thus, we analyzed these effects in the current study. In this study, the animal model of hind limb ischemia surgery in C57BL/6 mice was performed. Doppler ultrasound, immunohistochemical staining, and Western blotting were employed to analyze the situation of angiogenesis. In addition, human endothelial progenitor cells (EPCs) were used for in vitro studies to analyze the possible mechanisms. The results in the animal study showed that LIGHT injection inhibited angiogenesis in ischemic limbs. For the in vitro studies, LIGHT inhibited the expression of integrins and E-selectin; decreased migration and tube formation capabilities, mitochondrial respiration, and succinate dehydrogenase activity; and promoted senescence in EPCs. Western blotting revealed that the impairment of EPC function by LIGHT may be due to its effects on the proper functioning of the intracellular Akt signaling pathway, endothelial nitrite oxide synthase (eNOS), and mitochondrial respiration. In conclusion, LIGHT inhibits angiogenesis after tissue ischemia. This may be related to the clamped EPC function.
Keywords: LIGHT; endothelial progenitor cells; tumor necrosis factor superfamily 14.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study or in the collection, analysis or interpretation of the data.
Figures





Similar articles
-
CXCR7 Agonist TC14012 Improves Angiogenic Function of Endothelial Progenitor Cells via Activating Akt/eNOS Pathway and Promotes Ischemic Angiogenesis in Diabetic Limb Ischemia.Cardiovasc Drugs Ther. 2023 Oct;37(5):849-863. doi: 10.1007/s10557-022-07337-9. Epub 2022 Apr 26. Cardiovasc Drugs Ther. 2023. PMID: 35471717 Free PMC article.
-
C1q/Tumor Necrosis Factor-Related Protein-9 Is a Novel Vasculoprotective Cytokine That Restores High Glucose-Suppressed Endothelial Progenitor Cell Functions by Activating the Endothelial Nitric Oxide Synthase.J Am Heart Assoc. 2024 Feb 20;13(4):e030054. doi: 10.1161/JAHA.123.030054. Epub 2024 Feb 13. J Am Heart Assoc. 2024. PMID: 38348774 Free PMC article.
-
Inhalation of Fine Particulate Matter Impairs Endothelial Progenitor Cell Function Via Pulmonary Oxidative Stress.Arterioscler Thromb Vasc Biol. 2018 Jan;38(1):131-142. doi: 10.1161/ATVBAHA.117.309971. Epub 2017 Nov 30. Arterioscler Thromb Vasc Biol. 2018. PMID: 29191925 Free PMC article.
-
GroEL1, a heat shock protein 60 of Chlamydia pneumoniae, impairs neovascularization by decreasing endothelial progenitor cell function.PLoS One. 2013 Dec 23;8(12):e84731. doi: 10.1371/journal.pone.0084731. eCollection 2013. PLoS One. 2013. PMID: 24376840 Free PMC article.
-
Melatonin Improves Ischemia-Induced Circulation Recovery Impairment in Mice with Streptozotocin-Induced Diabetes by Improving the Endothelial Progenitor Cells Functioning.Int J Mol Sci. 2022 Aug 30;23(17):9839. doi: 10.3390/ijms23179839. Int J Mol Sci. 2022. PMID: 36077238 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

