RFRP-3 Influences Apoptosis and Steroidogenesis of Yak Cumulus Cells and Compromises Oocyte Meiotic Maturation and Subsequent Developmental Competence
- PMID: 37108163
- PMCID: PMC10138887
- DOI: 10.3390/ijms24087000
RFRP-3 Influences Apoptosis and Steroidogenesis of Yak Cumulus Cells and Compromises Oocyte Meiotic Maturation and Subsequent Developmental Competence
Abstract
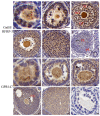
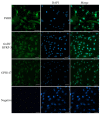
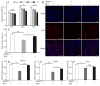
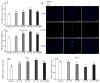
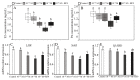
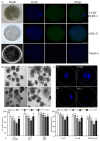
RF amide-related peptide 3 (RFRP-3), a mammalian ortholog of gonadotropin-inhibitory hormone (GnIH), is identified to be a novel inhibitory endogenous neurohormonal peptide that regulates mammalian reproduction by binding with specific G protein-coupled receptors (GPRs) in various species. Herein, our objectives were to explore the biological functions of exogenous RFRP-3 on the apoptosis and steroidogenesis of yak cumulus cells (CCs) and the developmental potential of yak oocytes. The spatiotemporal expression pattern and localization of GnIH/RFRP-3 and its receptor GPR147 were determined in follicles and CCs. The effects of RFRP-3 on the proliferation and apoptosis of yak CCs were initially estimated by EdU assay and TUNEL staining. We confirmed that high-dose (10-6 mol/L) RFRP-3 suppressed viability and increased the apoptotic rates, implying that RFRP-3 could repress proliferation and induce apoptosis. Subsequently, the concentrations of E2 and P4 were significantly lower with 10-6 mol/L RFRP-3 treatment than that of the control counterparts, which indicated that the steroidogenesis of CCs was impaired after RFRP-3 treatment. Compared with the control group, 10-6 mol/L RFRP-3 treatment decreased the maturation of yak oocytes efficiently and subsequent developmental potential. We sought to explore the potential mechanism of RFRP-3-induced apoptosis and steroidogenesis, so we observed the levels of apoptotic regulatory factors and hormone synthesis-related factors in yak CCs after RFRP-3 treatment. Our results indicated that RFRP-3 dose-dependently elevated the expression of apoptosis markers (Caspase and Bax), whereas the expression levels of steroidogenesis-related factors (LHR, StAR, 3β-HSD) were downregulated in a dose-dependent manner. However, all these effects were moderated by cotreatment with inhibitory RF9 of GPR147. These results demonstrated that RFRP-3 adjusted the expression of apoptotic and steroidogenic regulatory factors to induce apoptosis of CCs, probably through binding with its receptor GPR147, as well as compromised oocyte maturation and developmental potential. This research revealed the expression profiles of GnIH/RFRP-3 and GPR147 in yak CCs and supported a conserved inhibitory action on oocyte developmental competence.
Keywords: RFRP-3; apoptosis; cumulus cell; development; steroidogenesis.
Conflict of interest statement
The authors declare that the authors’ institution have no financial or other relationship with other people or organizations that may inappropriately influence the authors’ work.
Figures
Similar articles
-
Direct effects of RFRP-1, a mammalian GnIH ortholog, on ovarian activities of the cyclic mouse.Gen Comp Endocrinol. 2017 Oct 1;252:193-199. doi: 10.1016/j.ygcen.2017.06.024. Epub 2017 Jun 26. Gen Comp Endocrinol. 2017. PMID: 28658602
-
Identification of differential abundances of mRNA transcript in cumulus cells and CCND1 associated with yak oocyte developmental competence.Anim Reprod Sci. 2019 Sep;208:106135. doi: 10.1016/j.anireprosci.2019.106135. Epub 2019 Jul 23. Anim Reprod Sci. 2019. PMID: 31405458
-
The human gonadotropin-inhibitory hormone ortholog RFamide-related peptide-3 suppresses gonadotropin-induced progesterone production in human granulosa cells.Endocrinology. 2012 Jul;153(7):3435-45. doi: 10.1210/en.2012-1066. Epub 2012 Jun 12. Endocrinology. 2012. PMID: 22691551
-
RF-amide related peptide-3 (RFRP-3): a novel neuroendocrine regulator of energy homeostasis, metabolism, and reproduction.Mol Biol Rep. 2021 Feb;48(2):1837-1852. doi: 10.1007/s11033-021-06198-z. Epub 2021 Feb 10. Mol Biol Rep. 2021. PMID: 33566226 Review.
-
Regulation and Function of RFRP-3 (GnIH) Neurons during Postnatal Development.Front Endocrinol (Lausanne). 2015 Sep 24;6:150. doi: 10.3389/fendo.2015.00150. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 26441840 Free PMC article. Review.
Cited by
-
Dynamic Changes in Proteome during Yak Oocyte Maturation Analyzed Using iTRAQ Technology.Animals (Basel). 2023 Jun 23;13(13):2085. doi: 10.3390/ani13132085. Animals (Basel). 2023. PMID: 37443883 Free PMC article.
-
Factors Influencing the Maturation and Developmental Competence of Yak (Bos grunniens) Oocytes In Vitro.Genes (Basel). 2023 Sep 27;14(10):1882. doi: 10.3390/genes14101882. Genes (Basel). 2023. PMID: 37895231 Free PMC article. Review.
-
Impact of stress on male fertility: role of gonadotropin inhibitory hormone.Front Endocrinol (Lausanne). 2024 Jan 8;14:1329564. doi: 10.3389/fendo.2023.1329564. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38260147 Free PMC article. Review.
References
-
- Jia B.Y., Xiang D.C., Liu S.N., Zhang B., Shao Q.Y., Hong Q.H., Quan G.B., Wu G.Q. TMT-based quantitative proteomic analysis of cumulus cells derived from vitrified porcine immature oocytes following in vitro maturation. Theriogenology. 2020;152:8–17. doi: 10.1016/j.theriogenology.2020.04.025. - DOI - PubMed
-
- Dellaqua T.T., Vígaro R.A., Janini L.C.Z., Dal Canto M., Renzini M.M., Lodde V., Luciano A.M., Buratini J. Neuregulin 1 (NRG1) modulates oocyte nuclear maturation during IVM and improves post-IVF embryo development. Theriogenology. 2023;195:209–216. doi: 10.1016/j.theriogenology.2022.10.041. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 23ZDYF3118/the Key Research and Development Program of Sichuan Provincial Science and Technology Program
- SCCXTD-2022-13/the Special Project of Sichuan Beef Cattle Innovation Team of National Agricultural Industrial Technology System
- XM2023008/the Southwest Minzu University Double World-Class Project
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

