Re-Evaluating the Relevance of the Oxygen-Glucose Deprivation Model in Ischemic Stroke: The Example of Cdk Inhibition
- PMID: 37108171
- PMCID: PMC10138648
- DOI: 10.3390/ijms24087009
Re-Evaluating the Relevance of the Oxygen-Glucose Deprivation Model in Ischemic Stroke: The Example of Cdk Inhibition
Abstract
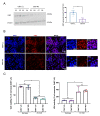
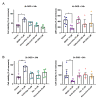
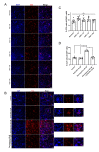
Previous research has shown that cyclin-dependent kinases (Cdks) that play physiological roles in cell cycle regulation become activated in post-mitotic neurons after ischemic stroke, resulting in apoptotic neuronal death. In this article, we report our results using the widely used oxygen-glucose deprivation (OGD) in vitro model of ischemic stroke on primary mouse cortical neurons to investigate whether Cdk7, as part of the Cdk-activating kinase (CAK) complex that activates cell cycle Cdks, might be a regulator of ischemic neuronal death and may potentially constitute a therapeutic target for neuroprotection. We found no evidence of neuroprotection with either pharmacological or genetic invalidation of Cdk7. Despite the well-established idea that apoptosis contributes to cell death in the ischemic penumbra, we also found no evidence of apoptosis in the OGD model. This could explain the absence of neuroprotection following Cdk7 invalidation in this model. Neurons exposed to OGD seem predisposed to die in an NMDA receptor-dependent manner that could not be prevented further downstream. Given the direct exposure of neurons to anoxia or severe hypoxia, it is questionable how relevant OGD is for modeling the ischemic penumbra. Due to remaining uncertainties about cell death after OGD, caution is warranted when using this in vitro model to identify new stroke therapies.
Keywords: OGD; cell culture; cortex; mouse; neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Silibinin activates AMP-activated protein kinase to protect neuronal cells from oxygen and glucose deprivation-re-oxygenation.Biochem Biophys Res Commun. 2014 Nov 14;454(2):313-9. doi: 10.1016/j.bbrc.2014.10.080. Epub 2014 Oct 22. Biochem Biophys Res Commun. 2014. PMID: 25450395
-
Critical role of extracellularly secreted neuronal pentraxin 1 in ischemic neuronal death.BMC Neurosci. 2014 Dec 20;15:133. doi: 10.1186/s12868-014-0133-3. BMC Neurosci. 2014. PMID: 25526743 Free PMC article.
-
Perilipin 5 protects against oxygen-glucose deprivation/reoxygenation-elicited neuronal damage by inhibiting oxidative stress and inflammatory injury via the Akt-GSK-3β-Nrf2 pathway.Int Immunopharmacol. 2022 Jul;108:108718. doi: 10.1016/j.intimp.2022.108718. Epub 2022 Mar 31. Int Immunopharmacol. 2022. PMID: 35367744
-
MicroRNA-152-3p protects neurons from oxygen-glucose-deprivation/reoxygenation-induced injury through upregulation of Nrf2/ARE antioxidant signaling by targeting PSD-93.Biochem Biophys Res Commun. 2019 Sep 10;517(1):69-76. doi: 10.1016/j.bbrc.2019.07.012. Epub 2019 Jul 17. Biochem Biophys Res Commun. 2019. PMID: 31326116
-
Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia.J Neurosci. 2005 Nov 2;25(44):10262-72. doi: 10.1523/JNEUROSCI.2818-05.2005. J Neurosci. 2005. PMID: 16267234 Free PMC article.
Cited by
-
Molecular Mechanisms and Pathophysiology of Acute Stroke: Recent Advances and Controversies.Curr Issues Mol Biol. 2024 Mar 27;46(4):2926-2930. doi: 10.3390/cimb46040182. Curr Issues Mol Biol. 2024. PMID: 38666912 Free PMC article.
-
Cerebral Hypoxia-Induced Molecular Alterations and Their Impact on the Physiology of Neurons and Dendritic Spines: A Comprehensive Review.Cell Mol Neurobiol. 2024 Aug 6;44(1):58. doi: 10.1007/s10571-024-01491-4. Cell Mol Neurobiol. 2024. PMID: 39105862 Free PMC article. Review.
References
-
- Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E.S., Baehrecke E.H., Blagosklonny M.V., El-Deiry W.S., Golstein P., Green D.R., et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

