RAGE-TLR4 Crosstalk Is the Key Mechanism by Which High Glucose Enhances the Lipopolysaccharide-Induced Inflammatory Response in Primary Bovine Alveolar Macrophages
- PMID: 37108174
- PMCID: PMC10138623
- DOI: 10.3390/ijms24087007
RAGE-TLR4 Crosstalk Is the Key Mechanism by Which High Glucose Enhances the Lipopolysaccharide-Induced Inflammatory Response in Primary Bovine Alveolar Macrophages
Abstract
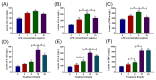
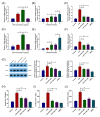
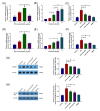
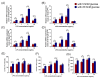
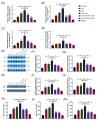
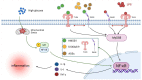
The receptor of advanced glycation end products (RAGE) and Toll-like receptor 4 (TLR4) are important receptors for inflammatory responses induced by high glucose (HG) and lipopolysaccharide (LPS) and show crosstalk phenomena in inflammatory responses. However, it is unknown whether RAGE and TLR4 can influence each other's expression through a crosstalk mechanism and whether the RAGE-TLR4 crosstalk related to the molecular mechanism of HG enhances the LPS-induced inflammatory response. In this study, the implications of LPS with multiple concentrations (0, 1, 5, and 10 μg/mL) at various treatment times (0, 3, 6, 12, and 24 h) in primary bovine alveolar macrophages (BAMs) were explored. The results showed that a 5 μg/mL LPS treatment at 12 h had the most significant increment on the pro-inflammatory cytokine interleukin 1β (IL-1β), IL-6, and tumor necrosis factor (TNF)-α levels in BAMs (p < 0.05) and that the levels of TLR4, RAGE, MyD88, and NF-κB p65 mRNA and protein expression were upregulated (p < 0.05). Then, the effect of LPS (5 μg/mL) and HG (25.5 mM) co-treatment in BAMs was explored. The results further showed that HG significantly enhanced the release of IL-1β, IL-6, and TNF-α caused by LPS in the supernatant (p < 0.01) and significantly increased the levels of RAGE, TLR4, MyD88, and NF-κB p65 mRNA and protein expression (p < 0.01). Pretreatment with FPS-ZM1 and TAK-242, the inhibitors of RAGE and TLR4, significantly alleviated the HG + LPS-induced increment of RAGE, TLR4, MyD88, and NF-κB p65 mRNA and protein expression in the presence of HG and LPS (p < 0.01). This study showed that RAGE and TLR4 affect each other's expression through crosstalk during the combined usage of HG and LPS and synergistically activate the MyD88/NF-κB signaling pathway to promote the release of pro-inflammatory cytokines in BAMs.
Keywords: TLR4–RAGE crosstalk; alveolar macrophages; glucose; inflammatory; lipopolysaccharide (LPS).
Conflict of interest statement
There are no conflicts of interest according to the authors.
Figures
Similar articles
-
Genistein attenuates LPS-induced inflammatory injury of rat dorsal root ganglion neuron via down-regulating HDAC6.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022 Jun 28;47(6):707-716. doi: 10.11817/j.issn.1672-7347.2022.210428. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 35837770 Free PMC article. Chinese, English.
-
[Alveolar macrophage TLR4/MyD88 signaling pathway contributes to ventilator-induced lung injury in rats].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015 Feb;31(2):182-5, 189. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015. PMID: 25652858 Chinese.
-
Anti-Inflammatory Activity of Adenosine 5'-Trisphosphate in Lipopolysaccharide-Stimulated Human Umbilical Vein Endothelial Cells Through Negative Regulation of Toll-Like Receptor MyD88 Signaling.DNA Cell Biol. 2019 Dec;38(12):1557-1563. doi: 10.1089/dna.2019.4773. Epub 2019 Oct 3. DNA Cell Biol. 2019. PMID: 31580158
-
An examination of the LPS-TLR4 immune response through the analysis of molecular structures and protein-protein interactions.Cell Commun Signal. 2025 Mar 18;23(1):142. doi: 10.1186/s12964-025-02149-4. Cell Commun Signal. 2025. PMID: 40102851 Free PMC article. Review.
-
Mechanism of ferroptosis in heart failure: The role of the RAGE/TLR4-JNK1/2 pathway in cardiomyocyte ferroptosis and intervention strategies.Ageing Res Rev. 2025 Jul;109:102770. doi: 10.1016/j.arr.2025.102770. Epub 2025 May 11. Ageing Res Rev. 2025. PMID: 40360081 Review.
Cited by
-
S100A9 and HMGB1 orchestrate MDSC-mediated immunosuppression in melanoma through TLR4 signaling.J Immunother Cancer. 2024 Sep 11;12(9):e009552. doi: 10.1136/jitc-2024-009552. J Immunother Cancer. 2024. PMID: 39266214 Free PMC article.
-
Reciprocal effect on lateral diffusion of receptor for advanced glycation endproducts and toll-like receptor 4 in the HEK293 cell membrane.Eur Biophys J. 2024 Aug;53(5-6):327-338. doi: 10.1007/s00249-024-01717-9. Epub 2024 Jul 27. Eur Biophys J. 2024. PMID: 39066956
-
Role of the Receptor for Advanced Glycation End Products (RAGE) and Its Ligands in Inflammatory Responses.Biomolecules. 2024 Dec 4;14(12):1550. doi: 10.3390/biom14121550. Biomolecules. 2024. PMID: 39766257 Free PMC article. Review.
-
Anserine reduces mortality in experimental sepsis by preventing methylglyoxal-induced capillary leakage.EBioMedicine. 2025 Apr;114:105644. doi: 10.1016/j.ebiom.2025.105644. Epub 2025 Mar 18. EBioMedicine. 2025. PMID: 40107203 Free PMC article.
-
Vinpocetine and Lactobacillus improve fatty liver in rats: role of adiponectin and gut microbiome.AMB Express. 2024 Aug 2;14(1):89. doi: 10.1186/s13568-024-01731-2. AMB Express. 2024. PMID: 39095672 Free PMC article.
References
-
- Baker E.H., Archer J.R.H., Srivastava S.A. Hyperglycemia, Lung Infection, and Inflammation. Clin. Pulm. Med. 2009;16:258–264. doi: 10.1097/CPM.0b013e3181b5d1df. - DOI
-
- Yu J., Shi J., Wang D., Dong S., Zhang Y., Wang M., Gong L., Fu Q., Liu D. Heme Oxygenase-1/Carbon Monoxide-regulated Mitochondrial Dynamic Equilibrium Contributes to the Attenuation of Endotoxin-induced Acute Lung Injury in Rats and in Lipopolysaccharide-activated Macrophages. Anesthesiology. 2016;125:1190–1201. doi: 10.1097/ALN.0000000000001333. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

