Selective Hepatic Cbs Knockout Aggravates Liver Damage, Endothelial Dysfunction and ROS Stress in Mice Fed a Western Diet
- PMID: 37108182
- PMCID: PMC10138434
- DOI: 10.3390/ijms24087019
Selective Hepatic Cbs Knockout Aggravates Liver Damage, Endothelial Dysfunction and ROS Stress in Mice Fed a Western Diet
Abstract
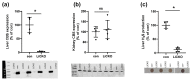
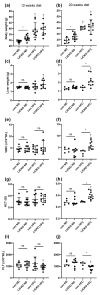
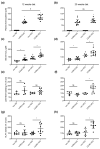
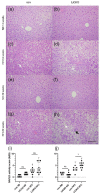
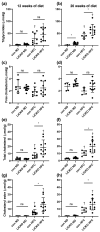
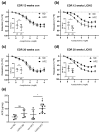
Cystathionine-β-synthase (CBS) is highly expressed in the liver, and deficiencies in Cbs lead to hyperhomocysteinemia (HHCy) and disturbed production of antioxidants such as hydrogen sulfide. We therefore hypothesized that liver-specific Cbs deficient (LiCKO) mice would be particularly susceptible to the development of non-alcoholic fatty liver disease (NAFLD). NAFLD was induced by a high-fat high-cholesterol (HFC) diet; LiCKO and controls were split into eight groups based on genotype (con, LiCKO), diet (normal diet, HFC), and diet duration (12 weeks, 20 weeks). LiCKO mice displayed intermediate to severe HHCy. Plasma H2O2 was increased by HFC, and further aggravated in LiCKO. LiCKO mice fed an HFC diet had heavier livers, increased lipid peroxidation, elevated ALAT, aggravated hepatic steatosis, and inflammation. LiCKO mice showed decreased L-carnitine in the liver, but this did not result in impaired fatty acid oxidation. Moreover, HFC-fed LiCKO mice demonstrated vascular and renal endothelial dysfunction. Liver and endothelial damage correlated significantly with systemic ROS status. In conclusion, this study demonstrates an important role for CBS in the liver in the development of NAFLD, which is most probably mediated through impaired defense against oxidative stress.
Keywords: NAFLD; cystathionine-β-synthase; high-fat diet; hydrogen sulfide; hyperhomocysteinemia; knockout mice; liver damage; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Tyrosol Attenuates High Fat Diet-Induced Hepatic Oxidative Stress: Potential Involvement of Cystathionine β-Synthase and Cystathionine γ-Lyase.Lipids. 2016 May;51(5):583-90. doi: 10.1007/s11745-015-4084-y. Epub 2015 Oct 30. Lipids. 2016. PMID: 26518313
-
Abnormal lipid metabolism in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia.J Biol Chem. 2004 Dec 17;279(51):52961-9. doi: 10.1074/jbc.M406820200. Epub 2004 Oct 4. J Biol Chem. 2004. PMID: 15466479
-
Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine beta-synthase-deficient mice.Am J Physiol Heart Circ Physiol. 2000 Sep;279(3):H970-5. doi: 10.1152/ajpheart.2000.279.3.H970. Am J Physiol Heart Circ Physiol. 2000. PMID: 10993757
-
Homozygous whole body Cbs knockout in adult mice features minimal pathology during ageing despite severe homocysteinemia.FASEB J. 2022 Apr;36(4):e22260. doi: 10.1096/fj.202101550R. FASEB J. 2022. PMID: 35315960
-
High fat diet-induced non alcoholic fatty liver disease in rats is associated with hyperhomocysteinemia caused by down regulation of the transsulphuration pathway.Lipids Health Dis. 2011 Apr 19;10:60. doi: 10.1186/1476-511X-10-60. Lipids Health Dis. 2011. PMID: 21504583 Free PMC article.
Cited by
-
Identification of hub genes significantly linked to tuberous sclerosis related-epilepsy and lipid metabolism via bioinformatics analysis.Front Neurol. 2024 Feb 14;15:1354062. doi: 10.3389/fneur.2024.1354062. eCollection 2024. Front Neurol. 2024. PMID: 38419709 Free PMC article.
-
Rodent model of metabolic dysfunction-associated fatty liver disease: a systematic review.J Gastroenterol Hepatol. 2025 Jan;40(1):48-66. doi: 10.1111/jgh.16749. Epub 2024 Sep 25. J Gastroenterol Hepatol. 2025. PMID: 39322221 Free PMC article.
-
Nitrous Oxide Abuse: Clinical Outcomes, Pharmacology, Pharmacokinetics, Toxicity and Impact on Metabolism.Toxics. 2023 Nov 28;11(12):962. doi: 10.3390/toxics11120962. Toxics. 2023. PMID: 38133363 Free PMC article. Review.
-
Gut Microbiota and Sinusoidal Vasoregulation in MASLD: A Portal Perspective.Metabolites. 2024 Jun 7;14(6):324. doi: 10.3390/metabo14060324. Metabolites. 2024. PMID: 38921459 Free PMC article. Review.
References
-
- Akahoshi N., Kobayashi C., Ishizaki Y., Izumi T., Himi T., Suematsu M., Ishii I. Genetic Background Conversion Ameliorates Semi-Lethality and Permits Behavioral Analyses in Cystathionine β-Synthase-Deficient Mice, an Animal Model for Hyperhomocysteinemia. Hum. Mol. Genet. 2008;17:1994–2005. doi: 10.1093/hmg/ddn097. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

