A New Gold(III) Complex, TGS 703, Shows Potent Anti-Inflammatory Activity in Colitis via the Enzymatic and Non-Enzymatic Antioxidant System-An In Vitro, In Silico, and In Vivo Study
- PMID: 37108188
- PMCID: PMC10138903
- DOI: 10.3390/ijms24087025
A New Gold(III) Complex, TGS 703, Shows Potent Anti-Inflammatory Activity in Colitis via the Enzymatic and Non-Enzymatic Antioxidant System-An In Vitro, In Silico, and In Vivo Study
Abstract
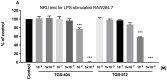
Inflammatory bowel diseases (IBD) and their main representatives, Crohn's disease and ulcerative colitis, are worldwide health-care problems with constantly increasing frequency and still not fully understood pathogenesis. IBD treatment involves drugs such as corticosteroids, derivatives of 5-aminosalicylic acid, thiopurines, and others, with the goal to achieve and maintain remission of the disease. Nowadays, as our knowledge about IBD is continually growing, more specific and effective therapies at the molecular level are wanted. In our study, we tested novel gold complexes and their potential effect on inflammation and IBD in vitro, in silico, and in vivo. A series of new gold(III) complexes (TGS 404, 512, 701, 702, and 703) were designed and screened in the in vitro inflammation studies. In silico modeling was used to study the gold complexes' structure vs. their activity and stability. Dextran sulphate sodium (DSS)-induced mouse model of colitis was employed to characterize the anti-inflammatory activity in vivo. Lipopolysaccharide (LPS)-stimulated RAW264.7 cell experiments proved the anti-inflammatory potential of all tested complexes. Selected on the bases of in vitro and in silico analyses, TGS 703 significantly alleviated inflammation in the DSS-induced mouse model of colitis, which was confirmed by a statistically significant decrease in the macro- and microscopic score of inflammation. The mechanism of action of TGS 703 was linked to the enzymatic and non-enzymatic antioxidant systems. TGS 703 and other gold(III) complexes present anti-inflammatory potential and may be applied therapeutically in the treatment of IBD.
Keywords: anti-inflammatory; gold; inflammatory bowel disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
New gold(III) complexes TGS 121, 404, and 702 show anti-tumor activity in colitis-induced colorectal cancer: an in vitro and in vivo study.Pharmacol Rep. 2024 Feb;76(1):127-139. doi: 10.1007/s43440-023-00558-1. Epub 2023 Dec 11. Pharmacol Rep. 2024. PMID: 38082190 Free PMC article.
-
New Class of Anti-Inflammatory Therapeutics Based on Gold (III) Complexes in Intestinal Inflammation-Proof of Concept Based on In Vitro and In Vivo Studies.Int J Mol Sci. 2021 Mar 18;22(6):3121. doi: 10.3390/ijms22063121. Int J Mol Sci. 2021. PMID: 33803793 Free PMC article.
-
Therapeutic Potential of Triptolide as an Anti-Inflammatory Agent in Dextran Sulfate Sodium-Induced Murine Experimental Colitis.Front Immunol. 2020 Nov 9;11:592084. doi: 10.3389/fimmu.2020.592084. eCollection 2020. Front Immunol. 2020. PMID: 33240279 Free PMC article.
-
PPARGC1A affects inflammatory responses in photodynamic therapy (PDT)-treated inflammatory bowel disease (IBD).Biochem Pharmacol. 2022 Aug;202:115119. doi: 10.1016/j.bcp.2022.115119. Epub 2022 Jun 3. Biochem Pharmacol. 2022. PMID: 35667414
-
Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis.World J Gastroenterol. 2017 Sep 7;23(33):6016-6029. doi: 10.3748/wjg.v23.i33.6016. World J Gastroenterol. 2017. PMID: 28970718 Free PMC article. Review.
Cited by
-
Metal-Based Drug-DNA Interactions and Analytical Determination Methods.Molecules. 2024 Sep 13;29(18):4361. doi: 10.3390/molecules29184361. Molecules. 2024. PMID: 39339356 Free PMC article. Review.
-
New gold(III) complexes TGS 121, 404, and 702 show anti-tumor activity in colitis-induced colorectal cancer: an in vitro and in vivo study.Pharmacol Rep. 2024 Feb;76(1):127-139. doi: 10.1007/s43440-023-00558-1. Epub 2023 Dec 11. Pharmacol Rep. 2024. PMID: 38082190 Free PMC article.
References
-
- Wang H.H., Su C.H., Wu Y.J., Lin C.A.J., Lee C.H., Shen J.L., Chan W.H., Chang W.H., Yeh H.I. Application of Gold in Biomedicine: Past, Present and Future. Int. J. Gerontol. 2012;6:1–4. doi: 10.1016/j.ijge.2011.09.015. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

