Vesicular Integral-Membrane Protein 36 Is Involved in the Selective Secretion of Fucosylated Proteins into Bile Duct-like Structures in HepG2 Cells
- PMID: 37108200
- PMCID: PMC10138374
- DOI: 10.3390/ijms24087037
Vesicular Integral-Membrane Protein 36 Is Involved in the Selective Secretion of Fucosylated Proteins into Bile Duct-like Structures in HepG2 Cells
Abstract
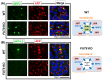
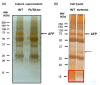
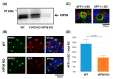
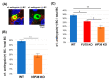
Fucosylated proteins are widely used as biomarkers of cancer and inflammation. Fucosylated alpha-fetoprotein (AFP-L3) is a specific biomarker for hepatocellular carcinoma. We previously showed that increases in serum AFP-L3 levels depend on increased expression of fucosylation-regulatory genes and abnormal transport of fucosylated proteins in cancer cells. In normal hepatocytes, fucosylated proteins are selectively secreted in the bile duct but not blood. In cases of cancer cells without cellular polarity, this selective secretion system is destroyed. Here, we aimed to identify cargo proteins involved in the selective secretion of fucosylated proteins, such as AFP-L3, into bile duct-like structures in HepG2 hepatoma cells, which have cellular polarity like, in part, normal hepatocytes. α1-6 Fucosyltransferase (FUT8) is a key enzyme to synthesize core fucose and produce AFP-L3. Firstly, we knocked out the FUT8 gene in HepG2 cells and investigated the effects on the secretion of AFP-L3. AFP-L3 accumulated in bile duct-like structures in HepG2 cells, and this phenomenon was diminished by FUT8 knockout, suggesting that HepG2 cells have cargo proteins for AFP-L3. To identify cargo proteins involved in the secretion of fucosylated proteins in HepG2 cells, immunoprecipitation and the proteomic Strep-tag system experiments followed by mass spectrometry analyses were performed. As a result of proteomic analysis, seven kinds of lectin-like molecules were identified, and we selected vesicular integral membrane protein gene VIP36 as a candidate of the cargo protein that interacts with the α1-6 fucosylation (core fucose) on N-glycan according to bibliographical consideration. Expectedly, the knockout of the VIP36 gene in HepG2 cells suppressed the secretion of AFP-L3 and other fucosylated proteins, such as fucosylated alpha-1 antitrypsin, into bile duct-like structures. We propose that VIP36 could be a cargo protein involved in the apical secretion of fucosylated proteins in HepG2 cells.
Keywords: AFP; HepG2; cargo protein; fucosylation; lectin.
Conflict of interest statement
Egashira and Fukagawa are employees of Sysmex Corporation. Other authors declare no conflict of interest for this article.
Figures

Similar articles
-
The enzymatic basis for the conversion of nonfucosylated to fucosylated alpha-fetoprotein by acyclic retinoid treatment in human hepatoma cells: activation of alpha1-6 fucosyltransferase.Tumour Biol. 2002 Jul-Aug;23(4):202-11. doi: 10.1159/000067253. Tumour Biol. 2002. PMID: 12499776
-
Analysis of polarized secretion of fucosylated alpha-fetoprotein in HepG2 cells.J Proteome Res. 2012 May 4;11(5):2798-806. doi: 10.1021/pr201154k. Epub 2012 Apr 17. J Proteome Res. 2012. PMID: 22483194
-
Fucosylation of N-glycans regulates the secretion of hepatic glycoproteins into bile ducts.J Biol Chem. 2006 Oct 6;281(40):29797-806. doi: 10.1074/jbc.M605697200. Epub 2006 Aug 9. J Biol Chem. 2006. PMID: 16899455
-
Huge pancreatic acinar cell carcinoma with high levels of AFP and fucosylated AFP (AFP-L3).Intern Med. 2012;51(11):1341-9. doi: 10.2169/internalmedicine.51.6536. Epub 2012 Jun 1. Intern Med. 2012. PMID: 22687839 Review.
-
The alpha1-6-fucosyltransferase gene and its biological significance.Biochim Biophys Acta. 1999 Dec 6;1473(1):9-20. doi: 10.1016/s0304-4165(99)00166-x. Biochim Biophys Acta. 1999. PMID: 10580126 Review.
Cited by
-
Regulation of Kv1.2 Redox-Sensitive Gating by the Transmembrane Lectin LMAN2.Function (Oxf). 2024 Nov 20;5(6):zqae041. doi: 10.1093/function/zqae041. Function (Oxf). 2024. PMID: 39264045 Free PMC article.
References
-
- Verhelst X., Dias A.M., Colombel J.F., Vermeire S., Van Vlierberghe H., Callewaert N., Pinho S.S. Protein Glycosylation as a Diagnostic and Prognostic Marker of Chronic Inflammatory Gastrointestinal and Liver Diseases. Gastroenterology. 2020;158:95–110. doi: 10.1053/j.gastro.2019.08.060. - DOI - PubMed
-
- Kim C.Y., Kim B.R., Lee S.S., Jeon D.H., Lee C.M., Kim W.S., Cho H.C., Kim J.J., Lee J.M., Kim H.J., et al. Clinical features of hepatitis B and C virus infections, with high α-fetoprotein levels but not hepatocellular carcinoma. Medicine. 2017;96:e5844. doi: 10.1097/MD.0000000000005844. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

