Glioblastoma Microenvironment and Invasiveness: New Insights and Therapeutic Targets
- PMID: 37108208
- PMCID: PMC10139189
- DOI: 10.3390/ijms24087047
Glioblastoma Microenvironment and Invasiveness: New Insights and Therapeutic Targets
Abstract
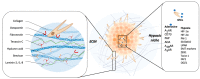
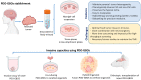
Glioblastoma (GBM) is the most common and malignant primary brain cancer in adults. Without treatment the mean patient survival is approximately 6 months, which can be extended to 15 months with the use of multimodal therapies. The low effectiveness of GBM therapies is mainly due to the tumor infiltration into the healthy brain tissue, which depends on GBM cells' interaction with the tumor microenvironment (TME). The interaction of GBM cells with the TME involves cellular components such as stem-like cells, glia, endothelial cells, and non-cellular components such as the extracellular matrix, enhanced hypoxia, and soluble factors such as adenosine, which promote GBM's invasiveness. However, here we highlight the role of 3D patient-derived glioblastoma organoids cultures as a new platform for study of the modeling of TME and invasiveness. In this review, the mechanisms involved in GBM-microenvironment interaction are described and discussed, proposing potential prognosis biomarkers and new therapeutic targets.
Keywords: brain tumors; glioblastoma; glioblastoma stem-like cells; invasiveness; tumor infiltration; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
- 1200885/Agencia Nacional de Investigación y Desarrollo
- 1211613/Agencia Nacional de Investigación y Desarrollo
- ICN09_016/ICN2021_045; former P09/016- F/Agencia Nacional de Investigación y Desarrollo
- 21181983/Agencia Nacional de Investigación y Desarrollo
- 1221253/Agencia Nacional de Investigación y Desarrollo
LinkOut - more resources
Full Text Sources
Medical

