The Forms of the Lectin Tff2 Differ in the Murine Stomach and Pancreas: Indications for Different Molecular Functions
- PMID: 37108221
- PMCID: PMC10138697
- DOI: 10.3390/ijms24087059
The Forms of the Lectin Tff2 Differ in the Murine Stomach and Pancreas: Indications for Different Molecular Functions
Abstract
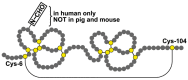
The lectin TFF2 belongs to the trefoil factor family (TFF). This polypeptide is typically co-secreted with the mucin MUC6 from gastric mucous neck cells, antral gland cells, and duodenal Brunner glands. Here, TFF2 fulfills a protective function by forming a high-molecular-mass complex with the MUC6, physically stabilizing the mucus barrier. In pigs and mice, and slightly in humans, TFF2 is also synthesized in the pancreas. Here, we investigated the murine stomach, pancreas, and duodenum by fast protein liquid chromatography (FPLC) and proteomics and identified different forms of Tff2. In both the stomach and duodenum, the predominant form is a high-molecular-mass complex with Muc6, whereas, in the pancreas, only low-molecular-mass monomeric Tff2 was detectable. We also investigated the expression of Tff2 and other selected genes in the stomach, pancreas, and the proximal, medial, and distal duodenum (RT-PCR analysis). The absence of the Tff2/Muc6 complex in the pancreas is due to a lack of Muc6. Based on its known motogenic, anti-apoptotic, and anti-inflammatory effects, we propose a protective receptor-mediated function of monomeric Tff2 for the pancreatic ductal epithelium. This view is supported by a report that a loss of Tff2 promotes the formation of pancreatic intraductal mucinous neoplasms.
Keywords: MUC6; TFF2; branching morphogenesis; lectin; mucin; pancreas; pancreatic cancer; pancreatic organogenesis; stomach; trefoil factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more (Review).Int J Oncol. 2015 Sep;47(3):806-16. doi: 10.3892/ijo.2015.3090. Epub 2015 Jul 17. Int J Oncol. 2015. PMID: 26201258 Review.
-
Loss of Trefoil Factor 2 From Pancreatic Duct Glands Promotes Formation of Intraductal Papillary Mucinous Neoplasms in Mice.Gastroenterology. 2016 Dec;151(6):1232-1244.e10. doi: 10.1053/j.gastro.2016.07.045. Epub 2016 Aug 12. Gastroenterology. 2016. PMID: 27523981 Free PMC article.
-
Commercial Porcine Gastric Mucin Preparations, also Used as Artificial Saliva, are a Rich Source for the Lectin TFF2: In Vitro Binding Studies.Chembiochem. 2018 Dec 18;19(24):2598-2608. doi: 10.1002/cbic.201800622. Epub 2018 Nov 27. Chembiochem. 2018. PMID: 30371971
-
Biosynthesis of gastrokine-2 in the human gastric mucosa: restricted spatial expression along the antral gland axis and differential interaction with TFF1, TFF2 and mucins.Cell Physiol Biochem. 2007;20(6):899-908. doi: 10.1159/000110450. Cell Physiol Biochem. 2007. PMID: 17982272
-
Trefoil Factor Family (TFF) Peptides and Their Diverse Molecular Functions in Mucus Barrier Protection and More: Changing the Paradigm.Int J Mol Sci. 2020 Jun 25;21(12):4535. doi: 10.3390/ijms21124535. Int J Mol Sci. 2020. PMID: 32630599 Free PMC article. Review.
Cited by
-
Expression Profiling along the Murine Intestine: Different Mucosal Protection Systems and Alterations in Tff1-Deficient Animals.Int J Mol Sci. 2023 Aug 11;24(16):12684. doi: 10.3390/ijms241612684. Int J Mol Sci. 2023. PMID: 37628863 Free PMC article.
-
Proteomics in pancreatic cancer.Biomark Res. 2025 Jul 6;13(1):93. doi: 10.1186/s40364-025-00805-y. Biomark Res. 2025. PMID: 40619414 Free PMC article. Review.
-
Different Forms of TFF3 in the Human Endocervix, including a Complex with IgG Fc Binding Protein (FCGBP), and Further Aspects of the Cervico-Vaginal Innate Immune Barrier.Int J Mol Sci. 2024 Feb 14;25(4):2287. doi: 10.3390/ijms25042287. Int J Mol Sci. 2024. PMID: 38396964 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

