In Vitro and In Vivo Effects of the Combination of Polypurine Reverse Hoogsteen Hairpins against HER-2 and Trastuzumab in Breast Cancer Cells
- PMID: 37108234
- PMCID: PMC10139100
- DOI: 10.3390/ijms24087073
In Vitro and In Vivo Effects of the Combination of Polypurine Reverse Hoogsteen Hairpins against HER-2 and Trastuzumab in Breast Cancer Cells
Abstract
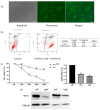
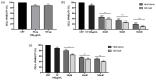
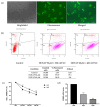
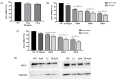
Therapeutic oligonucleotides are powerful tools for the inhibition of potential targets involved in cancer. We describe the effect of two Polypurine Reverse Hoogsteen (PPRH) hairpins directed against the ERBB2 gene, which is overexpressed in positive HER-2 breast tumors. The inhibition of their target was analyzed by cell viability and at the mRNA and protein levels. The combination of these specific PPRHs with trastuzumab was also explored in breast cancer cell lines, both in vitro and in vivo. PPRHs designed against two intronic sequences of the ERBB2 gene decreased the viability of SKBR-3 and MDA-MB-453 breast cancer cells. The decrease in cell viability was associated with a reduction in ERBB2 mRNA and protein levels. In combination with trastuzumab, PPRHs showed a synergic effect in vitro and reduced tumor growth in vivo. These results represent the preclinical proof of concept of PPRHs as a therapeutic tool for breast cancer.
Keywords: HER-2; PPRH; breast cancer; gene silencing; trastuzumab.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Polypurine reverse Hoogsteen hairpins as a gene therapy tool against survivin in human prostate cancer PC3 cells in vitro and in vivo.Biochem Pharmacol. 2013 Dec 1;86(11):1541-54. doi: 10.1016/j.bcp.2013.09.013. Epub 2013 Sep 23. Biochem Pharmacol. 2013. PMID: 24070653
-
Effect of Polypurine Reverse Hoogsteen Hairpins on Relevant Cancer Target Genes in Different Human Cell Lines.Nucleic Acid Ther. 2015 Aug;25(4):198-208. doi: 10.1089/nat.2015.0531. Epub 2015 Jun 4. Nucleic Acid Ther. 2015. PMID: 26042602
-
Coding polypurine hairpins cause target-induced cell death in breast cancer cells.Hum Gene Ther. 2011 Apr;22(4):451-63. doi: 10.1089/hum.2010.102. Epub 2011 Feb 16. Hum Gene Ther. 2011. PMID: 20942657
-
Polypurine Reverse Hoogsteen Hairpins as a Gene Silencing Tool for Cancer.Curr Med Chem. 2017;24(26):2809-2826. doi: 10.2174/0929867324666170301114127. Curr Med Chem. 2017. PMID: 28260512 Review.
-
Nucleic acids therapeutics using PolyPurine Reverse Hoogsteen hairpins.Biochem Pharmacol. 2021 Jul;189:114371. doi: 10.1016/j.bcp.2020.114371. Epub 2020 Dec 16. Biochem Pharmacol. 2021. PMID: 33338475 Review.
Cited by
-
Combinatorial Anti-Cancer Effect of Polypurine Reverse Hoogsteen Hairpins against KRAS and MYC Targeting in Prostate and Pancreatic Cancer Cell Lines.Genes (Basel). 2024 Oct 16;15(10):1332. doi: 10.3390/genes15101332. Genes (Basel). 2024. PMID: 39457457 Free PMC article.
-
Polypurine reverse hoogsteen hairpins as a therapeutic tool for SARS-CoV-2 infection.J Biol Chem. 2024 Nov;300(11):107884. doi: 10.1016/j.jbc.2024.107884. Epub 2024 Oct 11. J Biol Chem. 2024. PMID: 39395809 Free PMC article.
References
-
- Rodríguez L., Villalobos X., Dakhel S., Padilla L., Hervas R., Luis Hernández J., Ciudad C.J., Noe V. Polypurine reverse Hoogsteen hairpins as a gene therapy tool against survivin in human prostate cancer PC3 cells in vitro and in vivo n.d. Biochem. Pharmacol. 2013;86:1541–1554. doi: 10.1016/j.bcp.2013.09.013. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

